Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control
- PMID: 33476307
- PMCID: PMC7919731
- DOI: 10.1172/JCI138740
Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control
Abstract
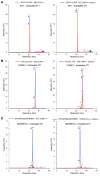
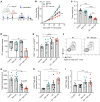
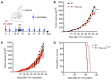
Neoantigens generated by somatic nonsynonymous mutations are key targets of tumor-specific T cells, but only a small number of mutations predicted to be immunogenic are presented by MHC molecules on cancer cells. Vaccination studies in mice and patients have shown that the majority of neoepitopes that elicit T cell responses fail to induce significant antitumor activity, for incompletely understood reasons. We report that radiotherapy upregulates the expression of genes containing immunogenic mutations in a poorly immunogenic mouse model of triple-negative breast cancer. Vaccination with neoepitopes encoded by these genes elicited CD8+ and CD4+ T cells that, whereas ineffective in preventing tumor growth, improved the therapeutic efficacy of radiotherapy. Mechanistically, neoantigen-specific CD8+ T cells preferentially killed irradiated tumor cells. Neoantigen-specific CD4+ T cells were required for the therapeutic efficacy of vaccination and acted by producing Th1 cytokines, killing irradiated tumor cells, and promoting epitope spread. Such a cytotoxic activity relied on the ability of radiation to upregulate class II MHC molecules as well as the death receptors FAS/CD95 and DR5 on the surface of tumor cells. These results provide proof-of-principle evidence that radiotherapy works in concert with neoantigen vaccination to improve tumor control.
Keywords: Antigen; Breast cancer; Immunology; Oncology; Radiation therapy.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

