Interferon regulatory factor 1 (IRF1) and anti-pathogen innate immune responses
- PMID: 33476326
- PMCID: PMC7819612
- DOI: 10.1371/journal.ppat.1009220
Interferon regulatory factor 1 (IRF1) and anti-pathogen innate immune responses
Abstract
The eponymous member of the interferon regulatory factor (IRF) family, IRF1, was originally identified as a nuclear factor that binds and activates the promoters of type I interferon genes. However, subsequent studies using genetic knockouts or RNAi-mediated depletion of IRF1 provide a much broader view, linking IRF1 to a wide range of functions in protection against invading pathogens. Conserved throughout vertebrate evolution, IRF1 has been shown in recent years to mediate constitutive as well as inducible host defenses against a variety of viruses. Fine-tuning of these ancient IRF1-mediated host defenses, and countering strategies by pathogens to disarm IRF1, play crucial roles in pathogenesis and determining the outcome of infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


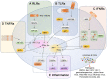


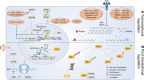
References
-
- Pine R, Decker T, Kessler DS, Levy DE, Darnell JE Jr. Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990;10(6):2448–57. 10.1128/mcb.10.6.2448 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

