Focal to bilateral tonic-clonic seizures are associated with widespread network abnormality in temporal lobe epilepsy
- PMID: 33476430
- PMCID: PMC8600951
- DOI: 10.1111/epi.16819
Focal to bilateral tonic-clonic seizures are associated with widespread network abnormality in temporal lobe epilepsy
Abstract
Objective: Our objective was to identify whether the whole-brain structural network alterations in patients with temporal lobe epilepsy (TLE) and focal to bilateral tonic-clonic seizures (FBTCS) differ from alterations in patients without FBTCS.
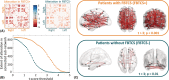
Methods: We dichotomized a cohort of 83 drug-resistant patients with TLE into those with and without FBTCS and compared each group to 29 healthy controls. For each subject, we used diffusion-weighted magnetic resonance imaging to construct whole-brain structural networks. First, we measured the extent of alterations by performing FBTCS-negative (FBTCS-) versus control and FBTCS-positive (FBTCS+) versus control comparisons, thereby delineating altered subnetworks of the whole-brain structural network. Second, by standardizing each patient's networks using control networks, we measured the subject-specific abnormality at every brain region in the network, thereby quantifying the spatial localization and the amount of abnormality in every patient.
Results: Both FBTCS+ and FBTCS- patient groups had altered subnetworks with reduced fractional anisotropy and increased mean diffusivity compared to controls. The altered subnetwork in FBTCS+ patients was more widespread than in FBTCS- patients (441 connections altered at t > 3, p < .001 in FBTCS+ compared to 21 connections altered at t > 3, p = .01 in FBTCS-). Significantly greater abnormalities-aggregated over the entire brain network as well as assessed at the resolution of individual brain areas-were present in FBTCS+ patients (p < .001, d = .82, 95% confidence interval = .32-1.3). In contrast, the fewer abnormalities present in FBTCS- patients were mainly localized to the temporal and frontal areas.
Significance: The whole-brain structural network is altered to a greater and more widespread extent in patients with TLE and FBTCS. We suggest that these abnormal networks may serve as an underlying structural basis or consequence of the greater seizure spread observed in FBTCS.
Keywords: connectome; diffusion MRI; drug-resistant epilepsy; network abnormality; node abnormality; secondary generalized seizures.
© 2021 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures



References
-
- Jobst BC, Williamson PD, Neuschwander TB, Darcey TM, Thadani VM, Roberts DW. Secondarily generalized seizures in mesial temporal epilepsy: clinical characteristics, lateralizing signs, and association with sleep‐wake cycle. Epilepsia. 2001;42:1279–87. - PubMed
-
- Lawn ND, Bamlet WR, Radhakrishnan K, O’Brien PC, So EL. Injuries due to seizures in persons with epilepsy: a population‐based study. Neurology. 2004;63:1565–70. - PubMed
-
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15:1075–88. - PubMed
-
- Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88:1674–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

