Plasma metabolomics of presymptomatic PSEN1-H163Y mutation carriers: a pilot study
- PMID: 33476461
- PMCID: PMC7951103
- DOI: 10.1002/acn3.51296
Plasma metabolomics of presymptomatic PSEN1-H163Y mutation carriers: a pilot study
Abstract
Background and objective: PSEN1-H163Y carriers, at the presymptomatic stage, have reduced 18 FDG-PET binding in the cerebrum of the brain (Scholl et al., Neurobiol Aging 32:1388-1399, 2011). This could imply dysfunctional energy metabolism in the brain. In this study, plasma of presymptomatic PSEN1 mutation carriers was analyzed to understand associated metabolic changes.
Methods: We analyzed plasma from noncarriers (NC, n = 8) and presymptomatic PSEN1-H163Y mutation carriers (MC, n = 6) via untargeted metabolomics using gas and liquid chromatography coupled with mass spectrometry, which identified 1199 metabolites. All the metabolites were compared between MC and NC using univariate analysis, as well as correlated with the ratio of Aβ1-42/A β 1-40 , using Spearman's correlation. Altered metabolites were subjected to Ingenuity Pathway Analysis (IPA).
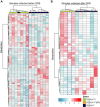
Results: Based on principal component analysis the plasma metabolite profiles were divided into dataset A and dataset B. In dataset A, when comparing between presymptomatic MC and NC, the levels of 79 different metabolites were altered. Out of 79, only 14 were annotated metabolites. In dataset B, 37 metabolites were significantly altered between presymptomatic MC and NC and nine metabolites were annotated. In both datasets, annotated metabolites represent amino acids, fatty acyls, bile acids, hexoses, purine nucleosides, carboxylic acids, and glycerophosphatidylcholine species. 1-docosapentaenoyl-GPC was positively correlated, uric acid and glucose were negatively correlated with the ratio of plasma Aβ1-42 /Aβ1-40 (P < 0.05).
Interpretation: This study finds dysregulated metabolite classes, which are changed before the disease symptom onset. Also, it provides an opportunity to compare with sporadic Alzheimer's Disease. Observed findings in this study need to be validated in a larger and independent Familial Alzheimer's Disease (FAD) cohort.
© 2021 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
None.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
