SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis
- PMID: 33476648
- PMCID: PMC7816624
- DOI: 10.1016/j.jbc.2021.100306
SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis
Abstract
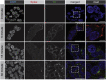
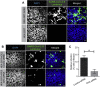
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of COVID-19, so understanding its biology and infection mechanisms is critical to facing this major medical challenge. SARS-CoV-2 is known to use its spike glycoprotein to interact with the cell surface as a first step in the infection process. As for other coronaviruses, it is likely that SARS-CoV-2 next undergoes endocytosis, but whether or not this is required for infectivity and the precise endocytic mechanism used are unknown. Using purified spike glycoprotein and lentivirus pseudotyped with spike glycoprotein, a common model of SARS-CoV-2 infectivity, we now demonstrate that after engagement with the plasma membrane, SARS-CoV-2 undergoes rapid, clathrin-mediated endocytosis. This suggests that transfer of viral RNA to the cell cytosol occurs from the lumen of the endosomal system. Importantly, we further demonstrate that knockdown of clathrin heavy chain, which blocks clathrin-mediated endocytosis, reduces viral infectivity. These discoveries reveal that SARS-CoV-2 uses clathrin-mediated endocytosis to gain access into cells and suggests that this process is a key aspect of virus infectivity.
Keywords: COVID-19; SARS-CoV-2; clathrin; dynamin; endocytosis; infection; virus entry.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
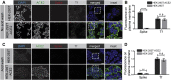

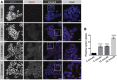
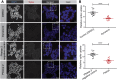

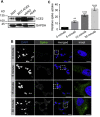

References
-
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. - PubMed
-
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

