Characterization of Properties, In Vitro and In Vivo Evaluation of Calcium Phosphate/Amino Acid Cements for Treatment of Osteochondral Defects
- PMID: 33477289
- PMCID: PMC7830446
- DOI: 10.3390/ma14020436
Characterization of Properties, In Vitro and In Vivo Evaluation of Calcium Phosphate/Amino Acid Cements for Treatment of Osteochondral Defects
Abstract
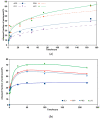
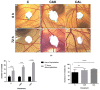
Novel calcium phosphate cements containing a mixture of four amino acids, glycine, proline, hydroxyproline and either lysine or arginine (CAL, CAK) were characterized and used for treatment of artificial osteochondral defects in knee. It was hypothesized that an enhanced concentration of extracellular collagen amino acids (in complex mixture), in connection with bone cement in defect sites, would support the healing of osteochondral defects with successful formation of hyaline cartilage and subchondral bone. Calcium phosphate cement mixtures were prepared by in situ reaction in a planetary ball mill at aseptic conditions and characterized. It was verified that about 30-60% of amino acids remained adsorbed on hydroxyapatite particles in cements and the addition of amino acids caused around 60% reduction in compressive strength and refinement of hydroxyapatite particles in their microstructure. The significant over-expression of osteogenic genes after the culture of osteoblasts was demonstrated in the cement extracts containing lysine and compared with other cements. The cement pastes were inserted into artificial osteochondral defects in the medial femoral condyle of pigs and, after 3 months post-surgery, tissues were analyzed macroscopically, histologically, immunohistochemically using MRI and X-ray methods. Analysis clearly showed the excellent healing process of artificial osteochondral defects in pigs after treatment with CAL and CAK cements without any inflammation, as well as formation of subchondral bone and hyaline cartilage morphologically and structurally identical to the original tissues. Good integration of the hyaline neocartilage with the surrounding tissue, as well as perfect interconnection between the neocartilage and new subchondral bone tissue, was demonstrated. Tissues were stable after 12 months' healing.
Keywords: amino acid; calcium phosphate cement; hyaline cartilage; osteochondral defect; pig model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Cole B.J., Harris J.D. Biologic Knee Reconstruction: A Surgeon’s Guide. 1st ed. SLACK, Inc.; Thorofare, NJ, USA: 2015. ISBN-13: 978-1617118166; ISBN-10: 1617118168.
-
- Puppi D., Chiellini F., Piras A.M., Chiellini E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010;35:403–440. doi: 10.1016/j.progpolymsci.2010.01.006. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

