Microbiome Signatures in a Fast- and Slow-Progressing Gastric Cancer Murine Model and Their Contribution to Gastric Carcinogenesis
- PMID: 33477306
- PMCID: PMC7829848
- DOI: 10.3390/microorganisms9010189
Microbiome Signatures in a Fast- and Slow-Progressing Gastric Cancer Murine Model and Their Contribution to Gastric Carcinogenesis
Abstract
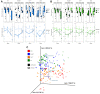
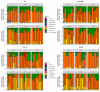
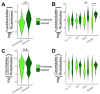
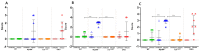
Gastric cancer is the third most common cause of death from cancer in the world and infection with Helicobacter pylori (H. pylori) is the main cause of gastric cancer. In addition to Helicobacter infection, the overall stomach microbiota has recently emerged as a potential factor in gastric cancer progression. Previously we had established that mice deficient in myeloid differentiation primary response gene 88 (MyD88, Myd88-/- ) rapidly progressed to neoplasia when infected with H. felis. Thus, in order to assess the role of the microbiota in this fast-progressing gastric cancer model we investigated changes of the gastric microbiome in mice with different genotypic backgrounds: wild type (WT), MyD88-deficient (Myd88-/- ), mice deficient in the Toll/interleukin-1 receptor (TIR) domain-containing adaptor-inducing interferon-β (TRIF, Trif Lps2), and MyD88- and TRIF-deficient (Myd88-/- /Trif Lps2, double knockout (DKO)) mice. We compared changes in alpha diversity, beta diversity, relative abundance, and log-fold differential of relative abundance ratios in uninfected and Helicobacter infected mice and studied their correlations with disease progression to gastric cancer in situ. We observed an overall reduction in microbial diversity post-infection with H. felis across all genotypes. Campylobacterales were observed in all infected mice, with marked reduction in abundance at 3 and 6 months in Myd88-/- mice. A sharp increase in Lactobacillales in infected Myd88-/- and DKO mice at 3 and 6 months was observed as compared to Trif Lps2 and WT mice, hinting at a possible role of these bacteria in gastric cancer progression. This was further reinforced upon comparison of Lactobacillales log-fold differentials with histological data, indicating that Lactobacillales are closely associated with Helicobacter infection and gastric cancer progression. Our study suggests that differences in genotypes could influence the stomach microbiome and make it more susceptible to the development of gastric cancer upon Helicobacter infection. Additionally, increase in Lactobacillales could contribute to faster development of gastric cancer and might serve as a potential biomarker for the fast progressing form of gastric cancer.
Keywords: Helicobacter; Lactobacillales; MyD88; TRIF; gastric cancer; microbiome.
Conflict of interest statement
The authors state they have no conflict of interest to declare.
Figures


References
-
- World Health Organization Cancer. WHO. [(accessed on 12 September 2018)]; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
-
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. [(accessed on 15 January 2021)];IARC Monogr. Eval. Carcinog. Risks Hum. 1994 61:1–241. Available online: https://www.ncbi.nlm.nih.gov/books/NBK487782/ - PMC - PubMed
-
- Aziz F., Chakarobaty A., Liu K., Yoshitomi H., Li X., Monts J., Xu G., Li Y., Bai R., Bode A.M., et al. Gastric tumorigenesis induced either by Helicobacter pylori infection or chronic alcohol consumption through IL-10 inhibition. Res. Sq. 2020 doi: 10.21203/rs.2.23065/v1. - DOI
-
- Majmudar K., Golemi I., Tafur A.J., Toro J.D., Visonà A., Falgá C., Sahuquillo J.C., Lorente M.A., Tufano A., Weinberg I., et al. RIETE Investigators. Outcomes after venous thromboembolism in patients with gastric cancer: Analysis of the RIETE Registry. Vasc. Med. 2020;25:210–217. doi: 10.1177/1358863X19893432. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

