In Vitro Evaluation of Polihexanide, Octenidine and NaClO/HClO-Based Antiseptics against Biofilm Formed by Wound Pathogens
- PMID: 33477349
- PMCID: PMC7830887
- DOI: 10.3390/membranes11010062
In Vitro Evaluation of Polihexanide, Octenidine and NaClO/HClO-Based Antiseptics against Biofilm Formed by Wound Pathogens
Abstract
Chronic wounds complicated with biofilm formed by pathogens remain one of the most significant challenges of contemporary medicine. The application of topical antiseptic solutions against wound biofilm has been gaining increasing interest among clinical practitioners and scientific researchers. This paper compares the activity of polyhexanide-, octenidine- and hypochlorite/hypochlorous acid-based antiseptics against biofilm formed by clinical strains of Candida albicans, Staphylococcus aureus and Pseudomonas aeruginosa. The analyses included both standard techniques utilizing polystyrene plates and self-designed biocellulose-based models in which a biofilm formed by pathogens was formed on an elastic, fibrinous surface covered with a fibroblast layer. The obtained results show high antibiofilm activity of polihexanide- and octenidine-based antiseptics and lack or weak antibiofilm activity of hypochlorite-based antiseptic of total chlorine content equal to 80 parts per million. The data presented in this paper indicate that polihexanide- or octenidine-based antiseptics are highly useful in the treatment of biofilm, while hypochlorite-based antiseptics with low chlorine content may be applied for wound rinsing but not when specific antibiofilm activity is required.
Keywords: hypochlorous acid; octenidine; polihexanide; sodium hypochlorite; wound biofilm.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

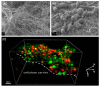
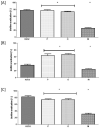
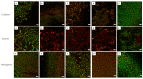
References
-
- Kadam S., Shai S., Shahane A., Kaushik K.S. Recent Advances in Non-Conventional Antimicrobial Approaches for Chronic Wound Biofilms: Have We Found the ‘Chink in the Armor’? [(accessed on 2 December 2020)];Biomedicines. 2019 7:35. doi: 10.3390/biomedicines7020035. Available online: https://www.mdpi.com/2227-9059/7/2/35#cite. - DOI - PMC - PubMed
-
- Mikulskis P., Hook A.L., Dundas A.A., Irvine D.J., Sanni O., Anderson D.G., Langer R., Alexander M.R., Williams P., Winkler D.A. Prediction of Broad-Spectrum Pathogen Attachment to Coating Materials for Biomedical Devices. ACS Appl. Mater. Interfaces. 2018;10:139–149. doi: 10.1021/acsami.7b14197. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

