Multiple-Monitor HPLC Assays for Rapid Process Development, In-Process Monitoring, and Validation of AAV Production and Purification
- PMID: 33477351
- PMCID: PMC7830902
- DOI: 10.3390/pharmaceutics13010113
Multiple-Monitor HPLC Assays for Rapid Process Development, In-Process Monitoring, and Validation of AAV Production and Purification
Abstract
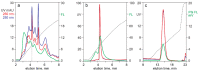
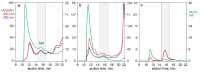
HPLC is established as a fast convenient analytical technology for characterizing the content of empty and full capsids in purified samples containing adeno-associated virus (AAV). UV-based monitoring unfortunately over-estimates the proportion of full capsids and offers little value for characterizing unpurified samples. The present study combines dual-wavelength UV monitoring with intrinsic fluorescence, extrinsic fluorescence, and light-scattering to extend the utility of HPLC for supporting development of therapeutic AAV-based drugs. Applications with anion exchange (AEC), cation exchange (CEC), and size exclusion chromatography (SEC) are presented. Intrinsic fluorescence increases sensitivity of AAV detection over UV and enables more objective estimation of empty and full capsid ratios by comparison of their respective peak areas. Light scattering enables identification of AAV capsids in complex samples, plus semiquantitative estimation of empty and full capsid ratios from relative peak areas of empty and full capsids. Extrinsic Picogreen fluorescence enables semiquantitative tracking of DNA with all HPLC methods at all stages of purification. It does not detect encapsidated DNA but reveals DNA associated principally with the exteriors of empty capsids. It also enables monitoring of host DNA contamination across chromatograms. These enhancements support many opportunities to improve characterization of raw materials and process intermediates, to accelerate process development, provide rapid in-process monitoring, and support process validation.
Keywords: AAV; HPLC; adeno-associated virus; empty capsids; extrinsic fluorescence; full capsids; in-process analysis; intrinsic fluorescence; light scattering; process development; validation.
Conflict of interest statement
The authors declare no conflict of interest. Sartorius had no role in the design of the study, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Giles A.R., Sims J.J., Turner K.B., Govindasamy L., Alvira M.R., Lock M., Wilson J.M. Deamidation of amino acids on the surface of adeno-associated virus capsids leads to charge heterogeneity and altered vector function. Mol. Ther. 2018;26:2848–2862. doi: 10.1016/j.ymthe.2018.09.013. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

