The Battle between Retroviruses and APOBEC3 Genes: Its Past and Present
- PMID: 33477360
- PMCID: PMC7830460
- DOI: 10.3390/v13010124
The Battle between Retroviruses and APOBEC3 Genes: Its Past and Present
Abstract
The APOBEC3 family of proteins in mammals consists of cellular cytosine deaminases and well-known restriction factors against retroviruses, including lentiviruses. APOBEC3 genes are highly amplified and diversified in mammals, suggesting that their evolution and diversification have been driven by conflicts with ancient viruses. At present, lentiviruses, including HIV, the causative agent of AIDS, are known to encode a viral protein called Vif to overcome the antiviral effects of the APOBEC3 proteins of their hosts. Recent studies have revealed that the acquisition of an anti-APOBEC3 ability by lentiviruses is a key step in achieving successful cross-species transmission. Here, we summarize the current knowledge of the interplay between mammalian APOBEC3 proteins and viral infections and introduce a scenario of the coevolution of mammalian APOBEC3 genes and viruses.
Keywords: APOBEC3; Vif; arms race; coevolution; gene diversification; lentivirus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
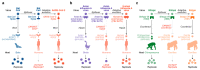

References
-
- WHO Coronavirus Disease 2019. [(accessed on 14 December 2020)];2020 Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

