The Comparative Clinical Performance of Four SARS-CoV-2 Rapid Antigen Tests and Their Correlation to Infectivity In Vitro
- PMID: 33477365
- PMCID: PMC7830733
- DOI: 10.3390/jcm10020328
The Comparative Clinical Performance of Four SARS-CoV-2 Rapid Antigen Tests and Their Correlation to Infectivity In Vitro
Abstract
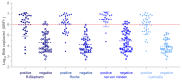
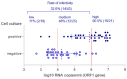
Due to globally rising numbers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, resources for real-time reverse-transcription polymerase chain reaction (rRT-PCR)-based testing have been exhausted. In order to meet the demands of testing and reduce transmission, SARS-CoV-2 antigen-detecting rapid diagnostic tests (Ag-RDTs) are being considered. These tests are fast, inexpensive, and simple to use, but whether they detect potentially infectious cases has not been well studied. We evaluated three lateral flow assays (RIDA®QUICK SARS-CoV-2 Antigen (R-Biopharm), SARS-CoV-2 Rapid Antigen Test (Roche)), and NADAL® COVID-19 Ag Test (Nal von Minden GmbH, Regensburg, Germany) and one microfluidic immunofluorescence assay (SARS-CoV-2 Ag Test (LumiraDx GmbH, Cologne, Germany)) using 100 clinical samples. Diagnostic rRT-PCR and cell culture testing as a marker for infectivity were performed in parallel. The overall Ag-RDT sensitivity for rRT-PCR-positive samples ranged from 24.3% to 50%. However, for samples with a viral load of more than 6 log10 RNA copies/mL (22/100), typically seen in infectious individuals, Ag-RDT positivity was between 81.8% and 100%. Only 51.6% (33/64) of the rRT-PCR-positive samples were infectious in cell culture. In contrast, three Ag-RDTs demonstrated a more significant correlation with cell culture infectivity (61.8-82.4%). Our findings suggest that large-scale SARS-CoV-2 Ag-RDT-based testing can be considered for detecting potentially infective individuals and reducing the virus spread.
Keywords: Ag-RDT; PCR; POCT; SARS-CoV-2; cell culture; infectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 14 December 2020)]; Available online: https://covid19.who.int/
-
- FIND SARS-CoV-2 Diagnostic Pipeline. [(accessed on 21 December 2020)]; Available online: https://www.finddx.org/covid-19/pipeline/?avance=Commercialized&type=Rap....
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

