Resistance to Antiandrogens in Prostate Cancer: Is It Inevitable, Intrinsic or Induced?
- PMID: 33477370
- PMCID: PMC7829888
- DOI: 10.3390/cancers13020327
Resistance to Antiandrogens in Prostate Cancer: Is It Inevitable, Intrinsic or Induced?
Abstract
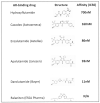
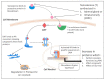
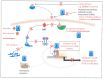
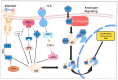
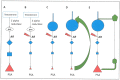
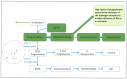
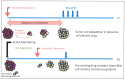
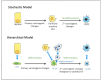
Increasingly sophisticated therapies for chemical castration dominate first-line treatments for locally advanced prostate cancer. However, androgen deprivation therapy (ADT) offers little prospect of a cure, as resistant tumors emerge rather rapidly, normally within 30 months. Cells have multiple mechanisms of resistance to even the most sophisticated drug regimes, and both tumor cell heterogeneity in prostate cancer and the multiple salvage pathways result in castration-resistant disease related genetically to the original hormone-naive cancer. The timing and mechanisms of cell death after ADT for prostate cancer are not well understood, and off-target effects after long-term ADT due to functional extra-prostatic expression of the androgen receptor protein are now increasingly being recorded. Our knowledge of how these widely used treatments fail at a biological level in patients is deficient. In this review, I will discuss whether there are pre-existing drug-resistant cells in a tumor mass, or whether resistance is induced/selected by the ADT. Equally, what is the cell of origin of this resistance, and does it differ from the treatment-naïve tumor cells by differentiation or dedifferentiation? Conflicting evidence also emerges from studies in the range of biological systems and species employed to answer this key question. It is only by improving our understanding of this aspect of treatment and not simply devising another new means of androgen inhibition that we can improve patient outcomes.
Keywords: androgen deprivation therapy: tumor resistance; androgens; model systems; prostate cancer.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Yang D.D., Mahal B.A., Muralidhar V., Martin N.E., Orio P.F., Mouw K.W., King M.T., Choueiri T.K., Trinh Q.-D., Hoffman K.E., et al. Androgen Deprivation Therapy and Overall Survival for Gleason 8 Versus Gleason 9–10 Prostate Cancer. Eur. Urol. 2019;75:35–41. doi: 10.1016/j.eururo.2018.08.033. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

