Molecular Changes Underlying Hypertrophic Scarring Following Burns Involve Specific Deregulations at All Wound Healing Stages (Inflammation, Proliferation and Maturation)
- PMID: 33477421
- PMCID: PMC7831008
- DOI: 10.3390/ijms22020897
Molecular Changes Underlying Hypertrophic Scarring Following Burns Involve Specific Deregulations at All Wound Healing Stages (Inflammation, Proliferation and Maturation)
Abstract
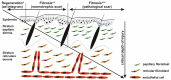
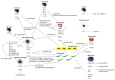
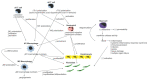
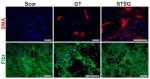
Excessive connective tissue accumulation, a hallmark of hypertrophic scaring, results in progressive deterioration of the structure and function of organs. It can also be seen during tumor growth and other fibroproliferative disorders. These processes result from a wide spectrum of cross-talks between mesenchymal, epithelial and inflammatory/immune cells that have not yet been fully understood. In the present review, we aimed to describe the molecular features of fibroblasts and their interactions with immune and epithelial cells and extracellular matrix. We also compared different types of fibroblasts and their roles in skin repair and regeneration following burn injury. In summary, here we briefly review molecular changes underlying hypertrophic scarring following burns throughout all basic wound healing stages, i.e. during inflammation, proliferation and maturation.
Keywords: burn; cell interaction; pathological scar; skin; stem cell; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bell L., McAdams T., Morgan R., Parshley P.F., Pike R.C., Riggs P., Carpenter J.E. Pruritus in burns: A descriptive study. J. Burn Care Rehabil. 1988;9:305–308. - PubMed
Publication types
MeSH terms
Grants and funding
- APVV-16-0207 and APVV-14-0731/Agentúra na Podporu Výskumu a Vývoja
- VEGA-1/0561/18/Vedecká Grantová Agentúra MŠVVaŠ SR a SAV
- PROGRES Q28 and Q37/Univerzita Karlova v Praze
- CZ.02.1.01/0.0/0.0/16_019/0000785 and ITMS2014+ 313011D103/European Regional Development Fund
- CA18103/European Cooperation in Science and Technology
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

