SARS-CoV-2 Cellular Infection and Therapeutic Opportunities: Lessons Learned from Ebola Virus
- PMID: 33477477
- PMCID: PMC7830673
- DOI: 10.3390/membranes11010064
SARS-CoV-2 Cellular Infection and Therapeutic Opportunities: Lessons Learned from Ebola Virus
Abstract
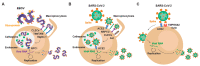
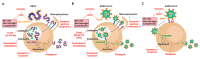
Viruses rely on the cellular machinery to replicate and propagate within newly infected individuals. Thus, viral entry into the host cell sets up the stage for productive infection and disease progression. Different viruses exploit distinct cellular receptors for viral entry; however, numerous viral internalization mechanisms are shared by very diverse viral families. Such is the case of Ebola virus (EBOV), which belongs to the filoviridae family, and the recently emerged coronavirus SARS-CoV-2. These two highly pathogenic viruses can exploit very similar endocytic routes to productively infect target cells. This convergence has sped up the experimental assessment of clinical therapies against SARS-CoV-2 previously found to be effective for EBOV, and facilitated their expedited clinical testing. Here we review how the viral entry processes and subsequent replication and egress strategies of EBOV and SARS-CoV-2 can overlap, and how our previous knowledge on antivirals, antibodies, and vaccines against EBOV has boosted the search for effective countermeasures against the new coronavirus. As preparedness is key to contain forthcoming pandemics, lessons learned over the years by combating life-threatening viruses should help us to quickly deploy effective tools against novel emerging viruses.
Keywords: Ebola virus; SARS-CoV-2; antibodies; antivirals; endocytosis; vaccines.
Conflict of interest statement
J.B. and J.C. are founders and shareholders of AlbaJuna Therapeutics, S.L.; B.C. is founder and shareholder of AlbaJuna Therapeutics, S.L. and AELIX Therapeutics, S.L. The authors declare no other competing interests.
Figures
References
-
- Perez-Zsolt D., Erkizia I., Pino M., García-Gallo M., Martin M.T., Benet S., Chojnacki J., Fernández-Figueras M.T., Guerrero D., Urrea V., et al. Anti-Siglec-1 Antibodies Block Ebola Viral Uptake and Decrease Cytoplasmic Viral Entry. Nat. Microbiol. 2019;4:1558–1570. doi: 10.1038/s41564-019-0453-2. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

