Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom
- PMID: 33477568
- PMCID: PMC7831143
- DOI: 10.3390/molecules26020483
Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom
Abstract
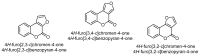
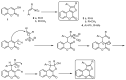
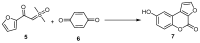
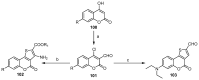
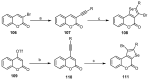
This review gives an up-to-date overview of the different ways (routes) to the synthesis of coumarin (benzopyrone)-fused, five-membered aromatic heterocycles with one heteroatom, built on the pyrone moiety. Covering 1966 to 2020.
Keywords: benzopyrones; coumarins; five-membered aromatic heterocycles; furan; pyrrole; selenophen; thiophene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
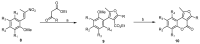
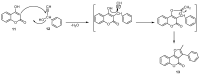
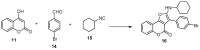
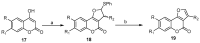
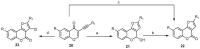
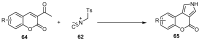
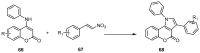
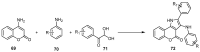
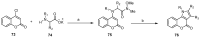
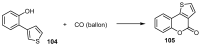
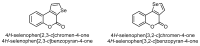
References
-
- Molnar M., Lončarić M., Kovač M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020;24:4–43. doi: 10.2174/1385272824666200120144305. - DOI
-
- Matos M.J., Santana L., Uriarte E., Abreu O.A., Molina E., Yordi E.G. Coumarins—An Important Class of Phytochemicals. Phytochem. Isol. Characterisation Role Hum. Health. 2015 doi: 10.5772/59982. - DOI
-
- Salem M.A., Helal M.H., Gouda M.A., Ammar Y.A., El-Gaby M.S.A., Abbas S.Y. An Overview on Synthetic Strategies to Coumarins. Synth. Commun. 2018;48:1534–1550. doi: 10.1080/00397911.2018.1455873. - DOI
-
- Irfan A., Rubab L., Rehman M.U., Anjum R., Ullah S., Marjana M., Qadeer S., Sana S. Coumarin Sulfonamide Derivatives: An Emerging Class of Therapeutic Agents. Heterocycl. Commun. 2020;26:46–59. doi: 10.1515/hc-2020-0008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

