Some Special Aspects of Liver Repair after Resection and Administration of Multipotent Stromal Cells in Experiment
- PMID: 33477612
- PMCID: PMC7831301
- DOI: 10.3390/life11010066
Some Special Aspects of Liver Repair after Resection and Administration of Multipotent Stromal Cells in Experiment
Abstract
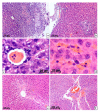
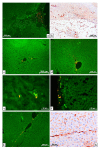
Changes in rat liver after resection and injection of autologous multipotent mesenchymal stromal cells of bone marrow origin (MSCs) transfected with the GFP gene and cell membranes stained with red-fluorescent lipophilic membrane dye were studied by light microscopy. It was found that after the introduction of MSCs into the damaged liver, their differentiation into any cells was not found. However, under the conditions of MSCs use, the number of neutrophils in the parenchyma normalizes earlier, and necrosis and hemorrhages disappear more quickly. It was concluded that the use of MSCs at liver resection for the rapid restoration of an organ is inappropriate, since the injected cells in vivo do not differentiate either into hepatocytes, into epithelial cells of bile capillaries, into endotheliocytes and pericytes of the vascular membranes, into fibroblasts of the scar or other connective tissue structures, or into any other cells present in the liver.
Keywords: liver; liver resection; macrophages; multipotent mesenchymal stromal cells; neutrophils.
Conflict of interest statement
The authors declare that they have no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures
Similar articles
-
Some Peculiarities of Local Distribution of Multipotent Mesenchymal Stromal Cells after Their Injection into Intact Muscle Tissue in Experiment.Bull Exp Biol Med. 2018 Mar;164(4):554-560. doi: 10.1007/s10517-018-4031-z. Epub 2018 Mar 4. Bull Exp Biol Med. 2018. PMID: 29504090
-
Possibility of Aggravation of Tissue Sclerosis after Injection of Multipotent Mesenchymal Stromal Cells Near the Forming Cicatrix in the Experiment.Bull Exp Biol Med. 2017 Aug;163(4):554-560. doi: 10.1007/s10517-017-3848-1. Epub 2017 Aug 29. Bull Exp Biol Med. 2017. PMID: 28853088
-
Injection of Multipotent Mesenchymal Stromal Cells as a Cause of Hemorrhages in the Regional Lymph Nodes: Experimental Study.Bull Exp Biol Med. 2018 Apr;164(6):784-789. doi: 10.1007/s10517-018-4080-3. Epub 2018 Apr 16. Bull Exp Biol Med. 2018. PMID: 29658074
-
Rationale for the potential use of mesenchymal stromal cells in liver transplantation.World J Gastroenterol. 2014 Nov 28;20(44):16418-32. doi: 10.3748/wjg.v20.i44.16418. World J Gastroenterol. 2014. PMID: 25469010 Free PMC article. Review.
-
Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors.Int J Biochem Cell Biol. 2014 Jan;46:90-102. doi: 10.1016/j.biocel.2013.11.006. Epub 2013 Nov 22. Int J Biochem Cell Biol. 2014. PMID: 24275096 Review.
Cited by
-
Possible Kidney Complications after Application of Cell Technologies for the Repair of the Resected Liver.Bull Exp Biol Med. 2023 May;175(1):138-143. doi: 10.1007/s10517-023-05825-y. Epub 2023 Jun 19. Bull Exp Biol Med. 2023. PMID: 37336807
-
Multipotent Stromal Cell Extracellular Vesicle Distribution in Distant Organs after Introduction into a Bone Tissue Defect of a Limb.Life (Basel). 2021 Apr 1;11(4):306. doi: 10.3390/life11040306. Life (Basel). 2021. PMID: 33916128 Free PMC article.
References
-
- Jung K., Kim Y., Heo Y., Lee J.C., Youn S., Moon J., Kim J., Kim T.Y., Kim B., Wang H. Management of severe blunt liver injuries by applying the damage control strategies with packing-oriented surgery: Experiences at a single institution in Korea. Hepatogastroenterology. 2015;62:410–416. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

