Dependence of Heat Transport in Solids on Length-Scale, Pressure, and Temperature: Implications for Mechanisms and Thermodynamics
- PMID: 33477677
- PMCID: PMC7831911
- DOI: 10.3390/ma14020449
Dependence of Heat Transport in Solids on Length-Scale, Pressure, and Temperature: Implications for Mechanisms and Thermodynamics
Abstract
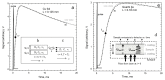
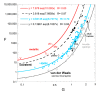
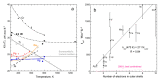
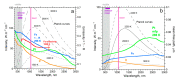
Accurate laser-flash measurements of thermal diffusivity (D) of diverse bulk solids at moderate temperature (T), with thickness L of ~0.03 to 10 mm, reveal that D(T) = D ∞(T)[1 - exp(-bL)]. When L is several mm, D ∞(T) = FT -G + HT, where F is constant, G is ~1 or 0, and H (for insulators) is ~0.001. The attenuation parameter b = 6.19D ∞ -0.477 at 298 K for electrical insulators, elements, and alloys. Dimensional analysis confirms that D → 0 as L → 0, which is consistent with heat diffusion, requiring a medium. Thermal conductivity (κ) behaves similarly, being proportional to D. Attenuation describing heat conduction signifies that light is the diffusing entity in solids. A radiative transfer model with 1 free parameter that represents a simplified absorption coefficient describes the complex form for κ(T) of solids, including its strong peak at cryogenic temperatures. Three parameters describe κ with a secondary peak and/or a high-T increase. The strong length dependence and experimental difficulties in diamond anvil studies have yielded problematic transport properties. Reliable low-pressure data on diverse thick samples reveal a new thermodynamic formula for specific heat (∂ln(cP )/∂P = -linear compressibility), which leads to ∂ln(κ)/∂P = linear compressibility + ∂lnα/∂P, where α is thermal expansivity. These formulae support that heat conduction in solids equals diffusion of light down the thermal gradient, since changing P alters the space occupied by matter, but not by light.
Keywords: heat; infrared absorption; laser flash analysis; length-scale physics; optical thickness; pressure; radiative diffusion; temperature; transport properties.
Conflict of interest statement
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures











Similar articles
-
Theory and Measurement of Heat Transport in Solids: How Rigidity and Spectral Properties Govern Behavior.Materials (Basel). 2024 Sep 11;17(18):4469. doi: 10.3390/ma17184469. Materials (Basel). 2024. PMID: 39336210 Free PMC article. Review.
-
Thermodynamic Relationships for Perfectly Elastic Solids Undergoing Steady-State Heat Flow.Materials (Basel). 2022 Apr 3;15(7):2638. doi: 10.3390/ma15072638. Materials (Basel). 2022. PMID: 35407969 Free PMC article.
-
Mantle values of thermal conductivity and the geotherm from phonon lifetimes.Science. 1999 Mar 12;283(5408):1699-706. doi: 10.1126/science.283.5408.1699. Science. 1999. PMID: 10073928
-
Pressure dependence of thermal transport properties.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9192-7. doi: 10.1073/pnas.0610734104. Epub 2007 Feb 13. Proc Natl Acad Sci U S A. 2007. PMID: 17299046 Free PMC article.
-
Temperature dependence of the contact angle of water: A review of research progress, theoretical understanding, and implications for boiling heat transfer.Adv Colloid Interface Sci. 2021 Feb;288:102339. doi: 10.1016/j.cis.2020.102339. Epub 2020 Dec 11. Adv Colloid Interface Sci. 2021. PMID: 33385775 Review.
Cited by
-
Theory and Measurement of Heat Transport in Solids: How Rigidity and Spectral Properties Govern Behavior.Materials (Basel). 2024 Sep 11;17(18):4469. doi: 10.3390/ma17184469. Materials (Basel). 2024. PMID: 39336210 Free PMC article. Review.
-
Thermodynamic Relationships for Perfectly Elastic Solids Undergoing Steady-State Heat Flow.Materials (Basel). 2022 Apr 3;15(7):2638. doi: 10.3390/ma15072638. Materials (Basel). 2022. PMID: 35407969 Free PMC article.
-
Proinflammatory polarization of engineered heat-inducible macrophages reprogram the tumor immune microenvironment during cancer immunotherapy.Nat Commun. 2024 Mar 15;15(1):2270. doi: 10.1038/s41467-024-46210-1. Nat Commun. 2024. PMID: 38491004 Free PMC article.
-
Examining Shape Dependence on Small Mild Steel Specimens during Heating Processes.Materials (Basel). 2024 Aug 7;17(16):3912. doi: 10.3390/ma17163912. Materials (Basel). 2024. PMID: 39203097 Free PMC article.
References
-
- Hofmeister A.M., Pertermann M., Branlund J.M. Thermal conductivity of the Earth. In: Schubert G., editor. Treatise in Geophysics. Volume 2. Elsevier; Amsterdam, The Netherlands: 2007. pp. 543–578.
-
- Kellett B.S. Transmission of radiation through glass in tank furnaces. J. Soc. Glass Tech. 1952;36:115–123.
-
- Berman R., Brock J.C. The effect of isotopes on lattice heat conduction. I. Lithium fluoride. Proc. R. Soc. Lond. A. 1965;289:46–65.
-
- Hofmeister A.M. Measurements, Mechanisms, and Models of Heat Transport. Elsevier; Amsterdam, The Netherlands: 2019.
-
- Chen G. Thermal conductivity and ballistic-phonon transport in the cross-plane direction of superlattices. Phys. Rev. B. 1998;57:14985. doi: 10.1103/PhysRevB.57.14958. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

