Effects of Alzheimer-Like Pathology on Homocysteine and Homocysteic Acid Levels-An Exploratory In Vivo Kinetic Study
- PMID: 33477684
- PMCID: PMC7831937
- DOI: 10.3390/ijms22020927
Effects of Alzheimer-Like Pathology on Homocysteine and Homocysteic Acid Levels-An Exploratory In Vivo Kinetic Study
Abstract
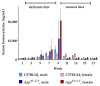
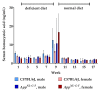
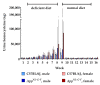
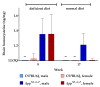
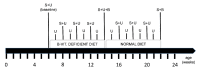
Hyperhomocysteinemia has been suggested potentially to contribute to a variety of pathologies, such as Alzheimer's disease (AD). While the impact of hyperhomocysteinemia on AD has been investigated extensively, there are scarce data on the effect of AD on hyperhomocysteinemia. The aim of this in vivo study was to investigate the kinetics of homocysteine (HCys) and homocysteic acid (HCA) and effects of AD-like pathology on the endogenous levels. The mice received a B-vitamin deficient diet for eight weeks, followed by the return to a balanced control diet for another eight weeks. Serum, urine, and brain tissues of AppNL-G-F knock-in and C57BL/6J wild type mice were analyzed for HCys and HCA using LC-MS/MS methods. Hyperhomocysteinemic levels were found in wild type and knock-in mice due to the consumption of the deficient diet for eight weeks, followed by a rapid normalization of the levels after the return to control chow. Hyperhomocysteinemic AppNL-G-F mice had significantly higher HCys in all matrices, but not HCA, compared to wild type control. Higher serum concentrations were associated with elevated levels in both the brain and in urine. Our findings confirm a significant impact of AD-like pathology on hyperhomocysteinemia in the AppNL-G-F mouse model. The immediate normalization of HCys and HCA after the supply of B-vitamins strengthens the idea of a B-vitamin intervention as a potentially preventive treatment option for HCys-related disorders such as AD.
Keywords: alzheimer disease; animal; disease models; hyperhomocysteinemia; vitamin B deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

