The Biology of Vasopressin
- PMID: 33477721
- PMCID: PMC7832310
- DOI: 10.3390/biomedicines9010089
The Biology of Vasopressin
Abstract
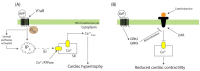
Vasopressins are evolutionarily conserved peptide hormones. Mammalian vasopressin functions systemically as an antidiuretic and regulator of blood and cardiac flow essential for adapting to terrestrial environments. Moreover, vasopressin acts centrally as a neurohormone involved in social and parental behavior and stress response. Vasopressin synthesis in several cell types, storage in intracellular vesicles, and release in response to physiological stimuli are highly regulated and mediated by three distinct G protein coupled receptors. Other receptors may bind or cross-bind vasopressin. Vasopressin is regulated spatially and temporally through transcriptional and post-transcriptional mechanisms, sex, tissue, and cell-specific receptor expression. Anomalies of vasopressin signaling have been observed in polycystic kidney disease, chronic heart failure, and neuropsychiatric conditions. Growing knowledge of the central biological roles of vasopressin has enabled pharmacological advances to treat these conditions by targeting defective systemic or central pathways utilizing specific agonists and antagonists.
Keywords: GPCRs; cardiac function; renal function; sex differences; social behavior; vasopressin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gamberi C., Hall K. Undergraduates can publish too! A case study of a scientific team writing assignment leading to publication. Int. J. Sci. Educ. 2019;41:48–63.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

