(-)-Epicatechin-An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation
- PMID: 33477734
- PMCID: PMC7832395
- DOI: 10.3390/antiox10010133
(-)-Epicatechin-An Important Contributor to the Antioxidant Activity of Japanese Knotweed Rhizome Bark Extract as Determined by Antioxidant Activity-Guided Fractionation
Abstract
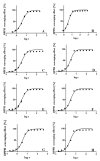
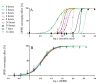
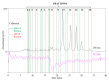
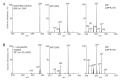
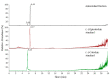
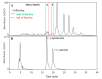
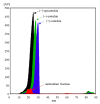
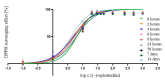
The antioxidant activities of Japanese knotweed rhizome bark extracts, prepared with eight different solvents or solvent mixtures (water, methanol, 80% methanol(aq), acetone, 70% acetone(aq), ethanol, 70% ethanol(aq), and 90% ethyl acetate(aq)), were determined using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging assay. Low half maximal inhibitory concentration (IC50) values (2.632-3.720 µg mL-1) for all the extracts were in the range of the IC50 value of the known antioxidant ascorbic acid at t0 (3.115 µg mL-1). Due to the highest extraction yield (~44%), 70% ethanol(aq) was selected for the preparation of the extract for further investigations. The IC50 value calculated for its antioxidant activity remained stable for at least 14 days, while the IC50 of ascorbic acid increased over time. The stability study showed that the container material was of great importance for the light-protected storage of the ascorbic acid(aq) solution in a refrigerator. Size exclusion-high-performance liquid chromatography (SEC-HPLC)-UV and reversed phase (RP)-HPLC-UV coupled with multistage mass spectrometry (MSn) were developed for fractionation of the 70% ethanol(aq) extract and for further compound identification, respectively. In the most potent antioxidant SEC fraction, determined using an on-line post-column SEC-HPLC-DPPH assay, epicatechin, resveratrol malonyl hexoside, and its in-source fragments (resveratrol and resveratrol acetyl hexoside) were tentatively identified by RP-HPLC-MSn. Moreover, epicatechin was additionally confirmed by two orthogonal methods, SEC-HPLC-UV and high-performance thin-layer chromatography (HPTLC) coupled with densitometry. Finally, the latter technique enabled the identification of (-)-epicatechin. (-)-Epicatechin demonstrated potent and stable time-dependent antioxidant activity (IC50 value ~1.5 µg mL-1) for at least 14 days.
Keywords: DPPH derivatization; DPPH test; Polygonum cuspidatum; Reynoutria; flavan-3-ols; invasive species; phenolic compounds; size-exclusion chromatography; stilbenes; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Balogh L. Japanese, Giant and Bohemian knotweed. In: Botta-Dukat Z., Balogh L., editors. The Most Important Invasive Plants in Hungary. HAS Institute of Ecology and Botany; Budapest, Hungary: 2008. pp. 13–33.
-
- Pogačnik L., Rogelj A., Ulrih N.P. Chemiluminescence method for evaluation of antioxidant capacities of different invasive knotweed species. Anal. Lett. 2015;49:350–363. doi: 10.1080/00032719.2014.979361. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

