Parvovirus-Based Combinatorial Immunotherapy: A Reinforced Therapeutic Strategy against Poor-Prognosis Solid Cancers
- PMID: 33477757
- PMCID: PMC7832409
- DOI: 10.3390/cancers13020342
Parvovirus-Based Combinatorial Immunotherapy: A Reinforced Therapeutic Strategy against Poor-Prognosis Solid Cancers
Abstract
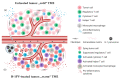
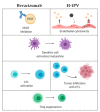
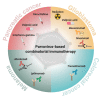
Resistance to anticancer treatments poses continuing challenges to oncology researchers and clinicians. The underlying mechanisms are complex and multifactorial. However, the immunologically "cold" tumor microenvironment (TME) has recently emerged as one of the critical players in cancer progression and therapeutic resistance. Therefore, TME modulation through induction of an immunological switch towards inflammation ("warming up") is among the leading approaches in modern oncology. Oncolytic viruses (OVs) are seen today not merely as tumor cell-killing (oncolytic) agents, but also as cancer therapeutics with multimodal antitumor action. Due to their intrinsic or engineered capacity for overcoming immune escape mechanisms, warming up the TME and promoting antitumor immune responses, OVs hold the potential for creating a proinflammatory background, which may in turn facilitate the action of other (immunomodulating) drugs. The latter provides the basis for the development of OV-based immunostimulatory anticancer combinations. This review deals with the smallest among all OVs, the H-1 parvovirus (H-1PV), and focuses on H-1PV-based combinatorial approaches, whose efficiency has been proven in preclinical and/or clinical settings. Special focus is given to cancer types with the most devastating impact on life expectancy that urgently call for novel therapies.
Keywords: colorectal cancer; combination therapy; glioblastoma; immunotherapy; melanoma; oncolytic; pancreatic cancer; parvovirus; tumor microenvironment.
Conflict of interest statement
A.A., T.F., J.R. and A.M. are holders of patents or patent applications related to H-1PV use for cancer therapeutic purposes. The parvovirus clinical trials (ParvOryx01, ParvOryx02) and compassionate H-1PV uses were financially supported by ORYX GmbH & Co. KG (Baldham, Germany). The funders had no role in the writing of the manuscript or in the decision to submit it for publication.
Figures

References
-
- Rommelaere J., Giese N., Cziepluch C., Cornelis J.J. Parvoviruses as anticancer agents. In: Sinkovics J.G., Horvath J.C., editors. Viral Therapy of Human Cancers. Marcel Dekker; New York, NY, USA: 2005. pp. 627–675.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

