ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients
- PMID: 33477776
- PMCID: PMC7832393
- DOI: 10.3390/cells10010186
ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients
Abstract
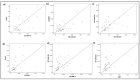
Endotheliopathy is suggested to be an important feature of COVID-19 in hospitalized patients. To determine whether endotheliopathy is involved in COVID-19-associated mortality, markers of endothelial damage were assessed in critically ill COVID-19 patients upon intensive care unit (ICU) admission. Thirty-eight critically ill COVID-19 patients were included in this observational study, 10 of whom died in the ICU. Endothelial biomarkers, including soluble (s)E-selectin, sP-selectin, angiopoietin 1 and 2 (Ang-1 and Ang-2, respectively), soluble intercellular adhesion molecule 1 (sICAM-1), vascular endothelial growth factor (VEGF), soluble vascular endothelial (VE)-cadherin, and von Willebrand factor (vWf), were measured upon ICU admission. The ICU cohort was subsequently divided into survivors and non-survivors; Kaplan-Meier analysis was used to explore associations between biomarkers and survival, while receiver operating characteristic (ROC) curves were generated to determine their potential prognostic value. sE-selectin, sP-selectin, Ang-2, and sICAM-1 were significantly elevated in ICU non-survivors compared to survivors, and also associated with a higher mortality probability in the Kaplan-Meier analysis. The prognostic values of sE-selectin, Ang-2, and sICAM-1 from the generated ROC curves were greater than 0.85. Hence, we conclude that in our cohort, ICU non-survivors had higher levels of specific endothelial markers compared to survivors. Elevated levels of these markers upon ICU admission could possibly predict mortality in COVID-19.
Keywords: COVID-19; angiopoietin; endotheliopathy; mortality; sE-selectin; sICAM-1; sP-selectin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

