Heat Stress Responses and Thermotolerance in Maize
- PMID: 33477941
- PMCID: PMC7833377
- DOI: 10.3390/ijms22020948
Heat Stress Responses and Thermotolerance in Maize
Abstract
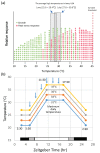
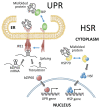
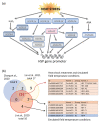
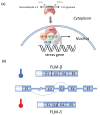
High temperatures causing heat stress disturb cellular homeostasis and impede growth and development in plants. Extensive agricultural losses are attributed to heat stress, often in combination with other stresses. Plants have evolved a variety of responses to heat stress to minimize damage and to protect themselves from further stress. A narrow temperature window separates growth from heat stress, and the range of temperatures conferring optimal growth often overlap with those producing heat stress. Heat stress induces a cytoplasmic heat stress response (HSR) in which heat shock transcription factors (HSFs) activate a constellation of genes encoding heat shock proteins (HSPs). Heat stress also induces the endoplasmic reticulum (ER)-localized unfolded protein response (UPR), which activates transcription factors that upregulate a different family of stress response genes. Heat stress also activates hormone responses and alternative RNA splicing, all of which may contribute to thermotolerance. Heat stress is often studied by subjecting plants to step increases in temperatures; however, more recent studies have demonstrated that heat shock responses occur under simulated field conditions in which temperatures are slowly ramped up to more moderate temperatures. Heat stress responses, assessed at a molecular level, could be used as traits for plant breeders to select for thermotolerance.
Keywords: heat stress; maize; post-transcriptional regulation; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Prasad P., Bheemanahalli R., Jagadish S.V.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crop. Res. 2017;200:114–121. doi: 10.1016/j.fcr.2016.09.024. - DOI
-
- Hoegh-Guldberg O., Jacob D., Taylor M., Bindi M., Brown S., Camilloni I., Diedhiou A., Djalante R., Ebi K., Engelbrecht F., et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. Intergovernmental Panel on Climate Change; Geneva, Switzerland: 2018. Chapter 3: Impacts of 1.5 °C global warming on natural and human systems; pp. 175–311. Specical Report.
-
- Obata T., Witt S., Lisec J., Palacios-Rojas N., Florez-Sarasa I., Yousfi S., Araus J.L., Cairns J.E., Fernie A.R. Metabolite profiles of maize leaves in drought, heat and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015;169:2665–2683. doi: 10.1104/pp.15.01164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

