Pro-Resolving FPR2 Agonists Regulate NADPH Oxidase-Dependent Phosphorylation of HSP27, OSR1, and MARCKS and Activation of the Respective Upstream Kinases
- PMID: 33477989
- PMCID: PMC7835750
- DOI: 10.3390/antiox10010134
Pro-Resolving FPR2 Agonists Regulate NADPH Oxidase-Dependent Phosphorylation of HSP27, OSR1, and MARCKS and Activation of the Respective Upstream Kinases
Abstract
Background: Formyl peptide receptor 2 (FPR2) is involved in the pathogenesis of chronic inflammatory diseases, being activated either by pro-resolving or proinflammatory ligands. FPR2-associated signal transduction pathways result in phosphorylation of several proteins and in NADPH oxidase activation. We, herein, investigated molecular mechanisms underlying phosphorylation of heat shock protein 27 (HSP27), oxidative stress responsive kinase 1 (OSR1), and myristolated alanine-rich C-kinase substrate (MARCKS) elicited by the pro-resolving FPR2 agonists WKYMVm and annexin A1 (ANXA1).
Methods: CaLu-6 cells or p22phoxCrispr/Cas9 double nickase CaLu-6 cells were incubated for 5 min with WKYMVm or ANXA1, in the presence or absence of NADPH oxidase inhibitors. Phosphorylation at specific serine residues of HSP27, OSR1, and MARCKS, as well as the respective upstream kinases activated by FPR2 stimulation was analysed.
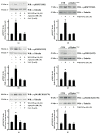
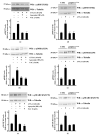
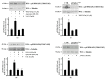
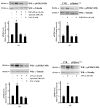
Results: Blockade of NADPH oxidase functions prevents WKYMVm- and ANXA1-induced HSP-27(Ser82), OSR1(Ser339) and MARCKS(Ser170) phosphorylation. Moreover, NADPH oxidase inhibitors prevent WKYMVm- and ANXA1-dependent activation of p38MAPK, PI3K and PKCδ, the kinases upstream to HSP-27, OSR1 and MARCKS, respectively. The same results were obtained in p22phoxCrispr/Cas9 cells.
Conclusions: FPR2 shows an immunomodulatory role by regulating proinflammatory and anti-inflammatory activities and NADPH oxidase is a key regulator of inflammatory pathways. The activation of NADPH oxidase-dependent pro-resolving downstream signals suggests that FPR2 signalling and NADPH oxidase could represent novel targets for inflammation therapeutic intervention.
Keywords: HSP-27; MARCKS (Myristolated Alanine-Rich C-Kinase Substrate); NADPH oxidase (Nicotinamide Adenine Dinucleotide Phosphate oxidase); OSR1 (Oxidative-Stress-Responsive kinase 1); annexin A1; formyl peptide receptors; inflammation; reactive oxygen species.
Conflict of interest statement
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

