New Prognostic Biomarkers in Metastatic Castration-Resistant Prostate Cancer
- PMID: 33478015
- PMCID: PMC7835961
- DOI: 10.3390/cells10010193
New Prognostic Biomarkers in Metastatic Castration-Resistant Prostate Cancer
Abstract
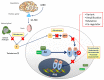
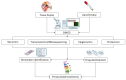
Prostate cancer is one of the most frequent cancers in men and is a common cause of cancer-related death. Despite significant progress in the diagnosis and treatment of this tumor, patients who relapse after radical treatments inevitably develop metastatic disease. Patient stratification is therefore key in this type of cancer, and there is an urgent need for prognostic biomarkers that can define patients' risk of cancer-related death. In the last 10 years, multiple prognostic factors have been identified and studied. Here, we review the literature available and discuss the most common aberrant genomic pathways in metastatic castration-resistant prostate cancer shown to have a prognostic relevance in this setting.
Keywords: DNA repair defects; PTEN; androgen receptor; prognostic biomarkers; prostate cancer.
Conflict of interest statement
Vincenza Conteduca has received speaker honoraria or travel support from Astellas, Janssen-Cilag and Sanofi-Aventis, and has received a consulting fee from Bayer. Nicole Brighi has received travel support from Ipsen, Novartis and Pfizer. Ugo De Giorgi has served as consultant/advisory board member for Astellas, Bayer, BMS, Ipsen, Janssen, Merck, Pfizer and Sanofi; has received travel support from BMS, Ipsen, Janssen and Pfizer; and has received research funding from AstraZeneca, Roche and Sanofi (Inst). The other The authors have no conflicts to declare.
Figures
References
-
- Arnold M., Karim-Kos H.E., Coebergh J.W., Byrnes G., Antilla A., Ferlay J., Renehan A.G., Forman D., Soerjomataram I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur. J. Cancer. 2015;51:1164–1187. doi: 10.1016/j.ejca.2013.09.002. - DOI - PubMed
-
- Luo J., Attard G., Balk S.P., Burnstein K., Cato L., Cherkasov A., De Bono J.S., Dong Y., Gao A.C., Gleave M., et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. Eur. Urol. 2018;73:715–723. doi: 10.1016/j.eururo.2017.11.038. - DOI - PMC - PubMed
-
- Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T., Henry A.M., Joniau S., Lam T.B., Mason M.D., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

