Parallel Recordings of Transmembrane hERG Channel Currents Based on Solvent-Free Lipid Bilayer Microarray
- PMID: 33478052
- PMCID: PMC7835820
- DOI: 10.3390/mi12010098
Parallel Recordings of Transmembrane hERG Channel Currents Based on Solvent-Free Lipid Bilayer Microarray
Abstract
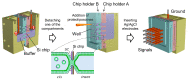
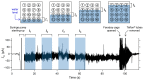
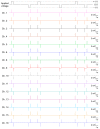
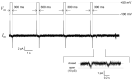
The reconstitution of ion-channel proteins in artificially formed bilayer lipid membranes (BLMs) forms a well-defined system for the functional analysis of ion channels and screening of the effects of drugs that act on these proteins. To improve the efficiency of the BLM reconstitution system, we report on a microarray of stable solvent-free BLMs formed in microfabricated silicon (Si) chips, where micro-apertures with well-defined nano- and micro-tapered edges were fabricated. Sixteen micro-wells were manufactured in a chamber made of Teflon®, and the Si chips were individually embedded in the respective wells as a recording site. Typically, 11 to 16 BLMs were simultaneously formed with an average BLM number of 13.1, which corresponded to a formation probability of 82%. Parallel recordings of ion-channel activities from multiple BLMs were successfully demonstrated using the human ether-a-go-go-related gene (hERG) potassium channel, of which the relation to arrhythmic side effects following drug treatment is well recognized.
Keywords: bilayer lipid membrane (BLM); human ether-a-go-go-related gene (hERG) channel; ion channel; microarray.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Miller C. Ion Channel Reconstitution. Plenum Publishing; New York, NY, USA: 1986.
-
- Tiefenauer L., Demarche S. Challenges in the development of functional assays of membrane proteins. Materials. 2012;5:2205–2242. doi: 10.3390/ma5112205. - DOI
-
- Kongsuphol P., Fang K.B., Ding Z. Lipid bilayer technologies in ion channel recordings and their potential in drug screening assay. Sens. Actuators B. 2013;185:530–542. doi: 10.1016/j.snb.2013.04.119. - DOI
-
- Imbrici P., Liantonio A., Camerino G.M., De Bellis M., Camerino C., Mele A., Giustino A., Pierno S., De Luca A., Tricarico D., et al. Therapeutic approaches to genetic ion channelopathies and perspectives in drug discovery. Front. Pharmacol. 2016;7:121. doi: 10.3389/fphar.2016.00121. - DOI - PMC - PubMed
Grants and funding
- JPMJCR14F3/Japan Science and Technology Agency
- 15H03822/Japan Society for the Promotion of Science
- 19H00846/Japan Society for the Promotion of Science
- 20K15148/Japan Society for the Promotion of Science
- N/A/Nation-wide Cooperative Research Projects, Research Institute of Electrical Communication, Tohoku University
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

