Glycyl-L-Prolyl-L-Glutamate Pseudotripeptides for Treatment of Alzheimer's Disease
- PMID: 33478054
- PMCID: PMC7835747
- DOI: 10.3390/biom11010126
Glycyl-L-Prolyl-L-Glutamate Pseudotripeptides for Treatment of Alzheimer's Disease
Abstract
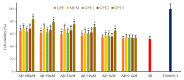
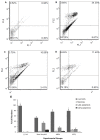
So far, there is no effective disease-modifying therapies for Alzheimer's Disease (AD) in clinical practice. In this context, glycine-L-proline-L-glutamate (GPE) and its analogs may open the way for developing a novel molecule for treating neurodegenerative disorders, including AD. In turn, this study was aimed to investigate the neuroprotective potentials exerted by three novel GPE peptidomimetics (GPE1, GPE2, and GPE3) using an in vitro AD model. Anti-Alzheimer potentials were determined using a wide array of techniques, such as measurements of mitochondrial viability (MTT) and lactate dehydrogenase (LDH) release assays, determination of acetylcholinesterase (AChE), α-secretase and β-secretase activities, comparisons of total antioxidant capacity (TAC) and total oxidative status (TOS) levels, flow cytometric and microscopic detection of apoptotic and necrotic neuronal death, and investigating gene expression responses via PCR arrays involving 64 critical genes related to 10 different pathways. Our analysis showed that GPE peptidomimetics modulate oxidative stress, ACh depletion, α-secretase inactivation, apoptotic, and necrotic cell death. In vitro results suggested that treatments with novel GPE analogs might be promising therapeutic agents for treatment and/or or prevention of AD.
Keywords: Alzheimer’s disease; gene expressions; glycine-proline-glutamate peptidomimetics; in vitro cell culture model; neurotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sara V.R., Carlsson-Skwirut C., Bergman T., Jörnvall H., Roberts P.J., Crawford M., Håkansson L.N., Civalero I., Nordberg A. Identification of Gly-Pro-Glu (GPE), the aminoterminal tripeptide of insulin-like growth factor 1 which is truncated in brain, as a novel neuroactive peptide. Biochem. Biophys. Res. Commun. 1989;165:766–771. doi: 10.1016/S0006-291X(89)80032-4. - DOI - PubMed
-
- Burgos-Ramos E., Martos-Moreno G.Á., López M.G., Herranz R., Aguado-Llera D., Egea J. The N-terminal tripeptide of insulin-like growth factor-I protects against β-amyloid-induced somatostatin depletion by calcium and glycogen synthase kinase 3β modulation. J. Neurochem. 2009;109:360–370. doi: 10.1111/j.1471-4159.2009.05980.x. - DOI - PubMed
-
- Guan J., Thomas G.B., Lin H., Mathai S., Bachelor D.C., George S. Neuroprotective effects of the N-terminal tripeptide of insulin-like growth factor-1, glycine-proline-glutamate (GPE) following intravenous infusion in hypoxic–ischemic adult rats. Neuropharmacology. 2004;47:892–903. doi: 10.1016/j.neuropharm.2004.07.002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

