Overexpression of Activated AMPK in the Anopheles stephensi Midgut Impacts Mosquito Metabolism, Reproduction and Plasmodium Resistance
- PMID: 33478058
- PMCID: PMC7835765
- DOI: 10.3390/genes12010119
Overexpression of Activated AMPK in the Anopheles stephensi Midgut Impacts Mosquito Metabolism, Reproduction and Plasmodium Resistance
Abstract
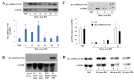
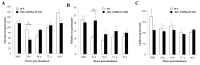
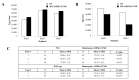
Mitochondrial integrity and homeostasis in the midgut are key factors controlling mosquito fitness and anti-pathogen resistance. Targeting genes that regulate mitochondrial dynamics represents a potential strategy for limiting mosquito-borne diseases. AMP-activated protein kinase (AMPK) is a key cellular energy sensor found in nearly all eukaryotic cells. When activated, AMPK inhibits anabolic pathways that consume ATP and activates catabolic processes that synthesize ATP. In this study, we overexpressed a truncated and constitutively active α-subunit of AMPK under the control of the midgut-specific carboxypeptidase promotor in the midgut of female Anopheles stephensi. As expected, AMPK overexpression in homozygous transgenic mosquitoes was associated with changes in nutrient storage and metabolism, decreasing glycogen levels at 24 h post-blood feeding when transgene expression was maximal, and concurrently increasing circulating trehalose at the same time point. When transgenic lines were challenged with Plasmodium falciparum, we observed a significant decrease in the prevalence and intensity of infection relative to wild type controls. Surprisingly, we did not observe a significant difference in the survival of adult mosquitoes fed either sugar only or both sugar and bloodmeals throughout adult life. This may be due to the limited period that the transgene was activated before homeostasis was restored. However, we did observe a significant decrease in egg production, suggesting that manipulation of AMPK activity in the mosquito midgut resulted in the re-allocation of resources away from egg production. In summary, this work identifies midgut AMPK activity as an important regulator of metabolism, reproduction, and innate immunity in An. stephensi, a highly invasive and important malaria vector species.
Keywords: AMPK; Anopheles stephensi; Plasmodium falciparum; malaria; metabolism; midgut; reproduction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Chan J.A., Zhang H., Roberts P.S., Jozwiak S., Wieslawa G., Lewin-Kowalik J., Kotulska K., Kwiatkowski D.J. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: Biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol. Exp. Neurol. 2004;63:1236–1242. doi: 10.1093/jnen/63.12.1236. - DOI - PubMed
-
- Horman S., Browne G.J., Krause U., Patel J.V., Vertommen D., Bertrand L., Lavoinne A., Hue L., Proud C.G., Rider M.H. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr. Biol. 2002;12:1419–1423. doi: 10.1016/S0960-9822(02)01077-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

