Surgical Treatment of Diabetic Foot Ulcers Complicated by Osteomyelitis with Gentamicin-Loaded Calcium Sulphate-Hydroxyapatite Biocomposite
- PMID: 33478085
- PMCID: PMC7835819
- DOI: 10.3390/jcm10020371
Surgical Treatment of Diabetic Foot Ulcers Complicated by Osteomyelitis with Gentamicin-Loaded Calcium Sulphate-Hydroxyapatite Biocomposite
Abstract
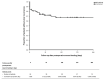
Diabetic foot ulcers, complicated by osteomyelitis, can be treated by surgical resection, dead space filling with gentamicin-loaded calcium sulphate-hydroxyapatite (CaS-HA) biocomposite, and closure of soft tissues and skin. To assess the feasibility of this treatment regimen, we conducted a multicenter retrospective cohort study of patients after failed conventional treatments. From 13 hospitals we included 64 patients with forefoot (n = 41 (64%)), midfoot (n = 14 (22%)), or hindfoot (n = 9 (14%)) ulcers complicated by osteomyelitis. Median follow-up was 43 (interquartile range, 20-61) weeks. We observed wound healing in 54 patients (84%) and treatment success (wound healing without ulcer recurrence) in 42 patients (66%). Treatment failures (no wound healing or ulcer recurrence) led to minor amputations in four patients (6%) and major amputations in seven patients (11%). Factors associated with treatment failures in univariable Cox regression analysis were gentamicin-resistant osteomyelitis (hazard ratio (HR), 3.847; 95%-confidence interval (CI), 1.065-13.899), hindfoot ulcers (HR, 3.624; 95%-CI, 1.187-11.060) and surgical procedures with gentamicin-loaded CaS-HA biocomposite that involved minor amputations (HR, 3.965; 95%-CI, 1.608-9.777). In this study of patients with diabetic foot ulcers, complicated by osteomyelitis, surgical treatment with gentamicin-loaded CaS-HA biocomposite was feasible and successful in 66% of patients. A prospective trial of this treatment regimen, based on a uniform treatment protocol, is required.
Keywords: diabetes mellitus; foot infections; foot ulcers; gentamicin-loaded calcium sulphate-hydroxyapatite biocomposite; osteomyelitis; surgery.
Conflict of interest statement
All authors declare that they have no conflicting financial or non-financial interests regarding the subject matter or the materials discussed in this article. While conducting the data collection in this study, we contacted the only firm (iMove medical B.V., Nieuwegein, the Netherlands) that distributed gentamicin-loaded calcium sulphate-hydroxyapatite biocomposite in the Netherlands during the study period, to obtain a complete list of Dutch hospitals in which treatments with this material were performed. We used this information to make contacts with the treating physicians in these hospitals, without involvement of iMove medical B.V. No agreements were made with IMove Medical B.V., and this firm had no further involvement or control in this study whatsoever.
Figures



References
-
- Prompers L., Huijberts M., Apelqvist J., Jude E., Piagessi A., Bakker K., Edmonds M., Holstein P., Jirkovska A., Mauricio D., et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25. doi: 10.1007/s00125-006-0491-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

