Human VAMP3 Suppresses or Negatively Regulates Bax Induced Apoptosis in Yeast
- PMID: 33478086
- PMCID: PMC7835773
- DOI: 10.3390/biomedicines9010095
Human VAMP3 Suppresses or Negatively Regulates Bax Induced Apoptosis in Yeast
Abstract
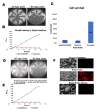
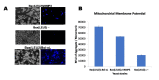
Apoptosis is an essential process that is regulated genetically and could lead to a serious disease condition if not well controlled. Bax is one of the main proapoptotic proteins and actively involved in programmed cell death. It has been suggested that Bax induced apoptosis in yeast could be obstructed by enhancing vesicular membrane trafficking. Plasma membrane proteins and lipid oxidation were reduced by a vesicle-associated membrane protein (VAMP) when expressed in yeast, suggesting its potential role in repairing membranes. Membrane integrity is crucial, as the loss of membrane integrity will result in the leakage of ions from mitochondria, and ultimately cell death due to overproduction of reactive oxygen species (ROS). Expression of Arabidopsis' VAMP has been linked to antiapoptosis activity. Since plant VAMP has been associated with antiapoptotic activities, this study investigates the possible participation of human VAMP3 in blocking human Bax mediated apoptosis. Some novel genes were identified to rescue Bax's proapoptotic effects, in a yeast-based human hippocampal cDNA library screen. VAMP3 (a gene code for proteins involved in protein secretion) gene was chosen for further study to confirm its role in inhibiting apoptosis. VAMP3 was coexpressed with a chromosomally integrated Bax gene expression cassette driven by the GAL1 promoter. The antiapoptotic proteins of the Bcl-2 family (Bcl xL) were known to negate the proapoptotic properties of Bax. However, the new gene (VAMP3) results show that novel antiapoptotic proteins can be identified using a yeast-based assay. The findings presented here show that human VAMP3 protein has antiapoptotic property and could abrogate Bax induced apoptosis (cell death).
Keywords: VAMP3 rescue Bax; Yeast and VAMP3; apoptosis; human VAMP3; yeast apoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Vesicle-associated membrane protein of Arabidopsis suppresses Bax-induced apoptosis in yeast downstream of oxidative burst.J Biol Chem. 2001 Dec 7;276(49):46284-9. doi: 10.1074/jbc.M107375200. Epub 2001 Sep 10. J Biol Chem. 2001. PMID: 11551960
-
Soybean ascorbate peroxidase suppresses Bax-induced apoptosis in yeast by inhibiting oxygen radical generation.Biochem Biophys Res Commun. 2002 Jan 11;290(1):457-62. doi: 10.1006/bbrc.2001.6208. Biochem Biophys Res Commun. 2002. PMID: 11779192
-
Bax-induced cell death of Arabidopsis is meditated through reactive oxygen-dependent and -independent processes.Plant Mol Biol. 2004 Sep;56(1):15-27. doi: 10.1007/s11103-004-3096-4. Plant Mol Biol. 2004. PMID: 15604726
-
Utilization of yeast to investigate the role of lipid oxidation in cell death.Antioxid Redox Signal. 2004 Apr;6(2):259-67. doi: 10.1089/152308604322899323. Antioxid Redox Signal. 2004. PMID: 15025927 Review.
-
Reconstituting the Mammalian Apoptotic Switch in Yeast.Genes (Basel). 2020 Jan 29;11(2):145. doi: 10.3390/genes11020145. Genes (Basel). 2020. PMID: 32013249 Free PMC article. Review.
Cited by
-
HCP5 prevents ubiquitination-mediated UTP3 degradation to inhibit apoptosis by activating c-Myc transcriptional activity.Mol Ther. 2023 Feb 1;31(2):552-568. doi: 10.1016/j.ymthe.2022.10.006. Epub 2022 Oct 17. Mol Ther. 2023. PMID: 36245126 Free PMC article.
-
miR-124 and VAMP3 Act Antagonistically in Human Neuroblastoma.Int J Mol Sci. 2023 Oct 4;24(19):14877. doi: 10.3390/ijms241914877. Int J Mol Sci. 2023. PMID: 37834325 Free PMC article.
-
Yeast as a tool to decipher the molecular mechanisms underlying the functions of Bcl-2 family.Explor Target Antitumor Ther. 2022;3(2):128-148. doi: 10.37349/etat.2022.00076. Epub 2022 Apr 2. Explor Target Antitumor Ther. 2022. PMID: 36046841 Free PMC article. Review.
-
VAMP proteins: molecular architects in disease pathogenesis and therapeutic innovation.Med Oncol. 2025 Aug 6;42(9):411. doi: 10.1007/s12032-025-02969-x. Med Oncol. 2025. PMID: 40770173 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

