Estimating a Stoichiometric Solid's Gibbs Free Energy Model by Means of a Constrained Evolutionary Strategy
- PMID: 33478133
- PMCID: PMC7835929
- DOI: 10.3390/ma14020471
Estimating a Stoichiometric Solid's Gibbs Free Energy Model by Means of a Constrained Evolutionary Strategy
Abstract
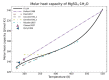
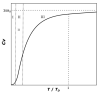
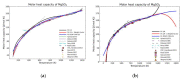
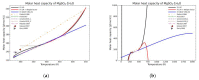
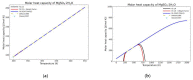
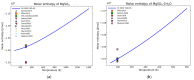
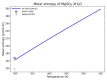
Modeling of thermodynamic properties, like heat capacities for stoichiometric solids, includes the treatment of different sources of data which may be inconsistent and diverse. In this work, an approach based on the covariance matrix adaptation evolution strategy (CMA-ES) is proposed and described as an alternative method for data treatment and fitting with the support of data source dependent weight factors and physical constraints. This is applied to a Gibb's Free Energy stoichiometric model for different magnesium sulfate hydrates by means of the NASA9 polynomial. Its behavior is proved by: (i) The comparison of the model to other standard methods for different heat capacity data, yielding a more plausible curve at high temperature ranges; (ii) the comparison of the fitted heat capacity values of MgSO4·7H2O against DSC measurements, resulting in a mean relative error of a 0.7% and a normalized root mean square deviation of 1.1%; and (iii) comparing the Van't Hoff and proposed Stoichiometric model vapor-solid equilibrium curves to different literature data for MgSO4·7H2O, MgSO4·6H2O, and MgSO4·1H2O, resulting in similar equilibrium values, especially for MgSO4·7H2O and MgSO4·6H2O. The results show good agreement with the employed data and confirm this method as a viable alternative for fitting complex physically constrained data sets, while being a potential approach for automatic data fitting of substance data.
Keywords: NASA9; constrained evolutionary strategy; data dispersion; data fitting; heat capacity; magnesium sulfate; salt hydrates; stoichiometric solid model; thermodynamic model; vapor-solid equilibrium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Moré J.J. Numerical Analysis. Springer; Berlin/Heidelberg, Germany: 1978. pp. 105–116.
-
- Stephan P., Schaber K., Stephan K., Mayinger F. Thermodynamik: Grundlagen und Technische Anwendungen-Band 2: Mehrstoffsysteme und Chemische Reaktionen. Springer; Berlin/Heidelberg, Germany: 2018.
-
- Sharma A., Tyagi V.V., Chen C.R., Buddhi D. Review on Thermal Energy Storage with Phase Change Materials and Applications. Renew. Sustain. Energy Rev. 2009;13:318–345. doi: 10.1016/j.rser.2007.10.005. - DOI
-
- Cabeza L.F., Castell A., Barreneche C., de Gracia A., Fernández A.I. Materials Used as PCM in Thermal Energy Storage in Buildings: A Review. Renew. Sustain. Energy Rev. 2011;15:1675–1695. doi: 10.1016/j.rser.2010.11.018. - DOI
-
- Donkers P.A.J., Sögütoglu L.C., Huinink H.P., Fischer H.R., Adan O.C.G. A Review of Salt Hydrates for Seasonal Heat Storage in Domestic Applications. Appl. Energy. 2017;199:45–68. doi: 10.1016/j.apenergy.2017.04.080. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

