An improved and efficient method of Agrobacterium syringe infiltration for transient transformation and its application in the elucidation of gene function in poplar
- PMID: 33478390
- PMCID: PMC7818742
- DOI: 10.1186/s12870-021-02833-w
An improved and efficient method of Agrobacterium syringe infiltration for transient transformation and its application in the elucidation of gene function in poplar
Abstract
Background: Forest trees have important economic and ecological value. As a model tree, poplar has played a significant role in elucidating the molecular mechanisms underlying tree biology. However, a lack of mutant libraries and time-consuming stable genetic transformation processes severely limit progress into the functional characterization of poplar genes. A convenient and fast transient transformation method is therefore needed to enhance progress on functional genomics in poplar.
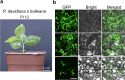
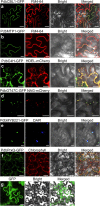
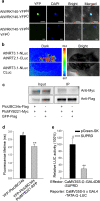
Methods: A total of 11 poplar clones were screened for amenability to syringe infiltration. Syringe infiltration was performed on the lower side of the leaves of young soil-grown plants. Transient expression was evaluated by visualizing the reporters β-glucuronidase (GUS) and green fluorescent protein (GFP). The experimental parameters of the syringe agroinfiltration were optimized based on the expression levels of the reporter luciferase (LUC). Stably transformed plants were regenerated from transiently transformed leaf explants through callus-induced organogenesis. The functions of Populus genes in secondary cell wall-thickening were characterized by visualizing lignin deposition therein after staining with basic fuchsin.
Results: We greatly improved the transient transformation efficiency of syringe Agrobacterium infiltration in poplar through screening for a suitable poplar clone from a variety of clones and optimizing the syringe infiltration procedure. The selected poplar clone, Populus davidiana × P. bolleana, is amenable to Agrobacterium syringe infiltration, as indicated by the easy diffusion of the bacterial suspension inside the leaf tissues. Using this technique, we localized a variety of poplar proteins in specific intracellular organelles and illustrated the protein-protein and protein-DNA interactions. The transiently transformed leaves could be used to generate stably transformed plants with high efficiency through callus induction and differentiation processes. Furthermore, transdifferentiation of the protoxylem-like vessel element and ectopic secondary wall thickening were induced in the agroinfiltrated leaves via the transient overexpression of genes associated with secondary wall formation.
Conclusions: The application of P. davidiana × P. bolleana in Agrobacterium syringe infiltration provides a foundation for the rapid and high-throughput functional characterization of Populus genes in intact poplar plants, including those involved in wood formation, and provides an effective alternative to Populus stable genetic transformation.
Keywords: Poplar; Secondary wall formation; Syringe Agrobacterium infiltration; Transgenic poplar; Transient expression.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Bradshaw H, Ceulemans R, Davis J, Stettler R. Emerging model systems in plant biology: poplar (Populus) as a model forest tree. J Plant Growth Regul. 2000;19(3):306–313. doi: 10.1007/s003440000030. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

