Short-Duration Prednisolone in Children with Nephrotic Syndrome Relapse: A Noninferiority Randomized Controlled Trial
- PMID: 33478976
- PMCID: PMC7863637
- DOI: 10.2215/CJN.06140420
Short-Duration Prednisolone in Children with Nephrotic Syndrome Relapse: A Noninferiority Randomized Controlled Trial
Abstract
Background and objectives: In children with nephrotic syndrome, steroids are the cornerstone of therapy for relapse. The adequate duration and dosage of steroids, however, have not been an active area of research, especially in children with infrequently relapsing nephrotic syndrome. This study investigated the efficacy of an abbreviated regimen for treatment of a relapse in this population.
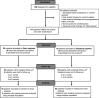
Design, setting, participants, & measurements: In a single-center, open-label, randomized controlled trial, we evaluated the efficacy of prednisolone as a "short regimen" (40 mg/m2 on alternate days for 2 weeks) compared with "standard regimen" (40 mg/m2 on alternate days for 4 weeks) for children aged 1-16 years who achieved remission of a relapse. The primary outcome was the proportion of children developing frequent relapses or steroid dependence at 12 months.
Results: A total of 117 patients were enrolled and randomized to short (55) or standard (62) regimen. Fourteen (24%) patients in standard regimen and 12 (23%) in short regimen developed frequent relapses or steroid dependence over a period of 1 year (risk difference, -1%; 95% confidence interval, -15 to 16; P=0.90). A large 95% confidence interval crossed the proposed noninferiority margin. In a time to event analysis, there was no significant difference in the proportion of children developing frequent relapses or steroid dependence and time to outcome between the two groups (hazard ratio, 1.01; 95% confidence interval, 0.83 to 1.23; P=0.98). Time to relapse, relapse rate, and steroid-related adverse events were similar in both groups. Cumulative steroid exposure was significantly lower in the short regimen (risk difference, -541 mg/m2; 95% confidence interval, -917 to -164 mg/m2; P<0.001).
Conclusions: In children with infrequently relapsing nephrotic syndrome, a short steroid treatment for relapse resulted in a similar proportion of patients developing frequent relapses or steroid dependence; however, noninferiority of a short regimen was not established.
Clinical trial registry name and registration number: CTRI/2015/11/006345.
Keywords: frequent relapses; infrequently relapsing nephrotic syndrome; nephrotic syndrome; prednisolone; short regimen.
Copyright © 2021 by the American Society of Nephrology.
Figures


Comment in
-
Steroid Regimen for Children with Nephrotic Syndrome Relapse.Clin J Am Soc Nephrol. 2021 Feb 8;16(2):179-181. doi: 10.2215/CJN.19201220. Epub 2021 Jan 21. Clin J Am Soc Nephrol. 2021. PMID: 33478977 Free PMC article. No abstract available.
References
-
- Churg J, Habib R, White RH: Pathology of the nephrotic syndrome in children: A report for the International Study of Kidney Disease in Children. Lancet 760: 1299–1302, 1970 - PubMed
-
- Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, Kalaivani M, Hari P, Bagga A: Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int 87: 217–224, 2015 - PubMed
-
- Ehrich JH, Brodehl J; Arbeitsgemeinschaft für Pädiatrische Nephrologie: Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Eur J Pediatr 152: 357–361, 1993 - PubMed
-
- Hallstrom A, Davis K: Imbalance in treatment assignments in stratified blocked randomization. Control Clin Trials 9: 375–382, 1988 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

