Model-informed COVID-19 vaccine prioritization strategies by age and serostatus
- PMID: 33479118
- PMCID: PMC7963218
- DOI: 10.1126/science.abe6959
Model-informed COVID-19 vaccine prioritization strategies by age and serostatus
Abstract
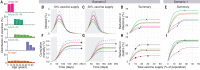
Limited initial supply of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine raises the question of how to prioritize available doses. We used a mathematical model to compare five age-stratified prioritization strategies. A highly effective transmission-blocking vaccine prioritized to adults ages 20 to 49 years minimized cumulative incidence, but mortality and years of life lost were minimized in most scenarios when the vaccine was prioritized to adults greater than 60 years old. Use of individual-level serological tests to redirect doses to seronegative individuals improved the marginal impact of each dose while potentially reducing existing inequities in COVID-19 impact. Although maximum impact prioritization strategies were broadly consistent across countries, transmission rates, vaccination rollout speeds, and estimates of naturally acquired immunity, this framework can be used to compare impacts of prioritization strategies across contexts.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


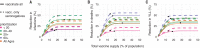
Update of
-
Model-informed COVID-19 vaccine prioritization strategies by age and serostatus.medRxiv [Preprint]. 2021 Jan 8:2020.09.08.20190629. doi: 10.1101/2020.09.08.20190629. medRxiv. 2021. Update in: Science. 2021 Feb 26;371(6532):916-921. doi: 10.1126/science.abe6959. PMID: 33330882 Free PMC article. Updated. Preprint.
Comment in
-
Optimizing age-specific vaccination.Science. 2021 Feb 26;371(6532):890-891. doi: 10.1126/science.abg2334. Epub 2021 Jan 21. Science. 2021. PMID: 33479122 No abstract available.
References
-
- Johns Hopkins University Coronavirus Research Center, COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University (2020); https://coronavirus.jhu.edu/map.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

