Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation
- PMID: 33479166
- PMCID: PMC8017934
- DOI: 10.1073/pnas.2017715118
Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation
Abstract
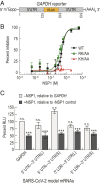
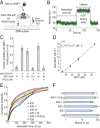
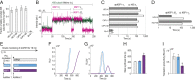
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a beta-CoV that recently emerged as a human pathogen and is the causative agent of the COVID-19 pandemic. A molecular framework of how the virus manipulates host cellular machinery to facilitate infection remains unclear. Here, we focus on SARS-CoV-2 NSP1, which is proposed to be a virulence factor that inhibits protein synthesis by directly binding the human ribosome. We demonstrate biochemically that NSP1 inhibits translation of model human and SARS-CoV-2 messenger RNAs (mRNAs). NSP1 specifically binds to the small (40S) ribosomal subunit, which is required for translation inhibition. Using single-molecule fluorescence assays to monitor NSP1-40S subunit binding in real time, we determine that eukaryotic translation initiation factors (eIFs) allosterically modulate the interaction of NSP1 with ribosomal preinitiation complexes in the absence of mRNA. We further elucidate that NSP1 competes with RNA segments downstream of the start codon to bind the 40S subunit and that the protein is unable to associate rapidly with 80S ribosomes assembled on an mRNA. Collectively, our findings support a model where NSP1 proteins from viruses in at least two subgenera of beta-CoVs associate with the open head conformation of the 40S subunit to inhibit an early step of translation, by preventing accommodation of mRNA within the entry channel.
Keywords: NSP1; SARS-CoV-2; eukaryotic translation initiation; human ribosome; single-molecule fluorescence.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

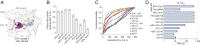

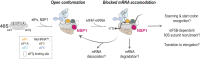
References
-
- Drosten C., et al., Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976 (2003). - PubMed
-
- Zaki A. M., van Boheemen S., Bestebroer T. M., Osterhaus A. D. M. E., Fouchier R. A. M., Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

