The human O-GlcNAcome database and meta-analysis
- PMID: 33479245
- PMCID: PMC7820439
- DOI: 10.1038/s41597-021-00810-4
The human O-GlcNAcome database and meta-analysis
Abstract
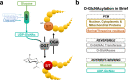
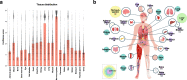
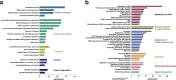
Over the past 35 years, ~1700 articles have characterized protein O-GlcNAcylation. Found in almost all living organisms, this post-translational modification of serine and threonine residues is highly conserved and key to biological processes. With half of the primary research articles using human models, the O-GlcNAcome recently reached a milestone of 5000 human proteins identified. Herein, we provide an extensive inventory of human O-GlcNAcylated proteins, their O-GlcNAc sites, identification methods, and corresponding references ( www.oglcnac.mcw.edu ). In the absence of a comprehensive online resource for O-GlcNAcylated proteins, this list serves as the only database of O-GlcNAcylated proteins. Based on the thorough analysis of the amino acid sequence surrounding 7002 O-GlcNAc sites, we progress toward a more robust semi-consensus sequence for O-GlcNAcylation. Moreover, we offer a comprehensive meta-analysis of human O-GlcNAcylated proteins for protein domains, cellular and tissue distribution, and pathways in health and diseases, reinforcing that O-GlcNAcylation is a master regulator of cell signaling, equal to the widely studied phosphorylation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

