COVID-19 vaccines: where we stand and challenges ahead
- PMID: 33479399
- PMCID: PMC7818063
- DOI: 10.1038/s41418-020-00720-9
COVID-19 vaccines: where we stand and challenges ahead
Abstract
In the eleven months elapsed since the identification of the SARS-CoV-2 virus and its genome, an exceptional effort by the scientific community has led to the development of over 300 vaccine projects. Over 40 are now undergoing clinical evaluation, ten of these are in Phase III clinical trials, three of them have ended Phase III with positive results. A few of these new vaccines are being approved for emergency use. Existing data suggest that new vaccine candidates may be instrumental in protecting individuals and reducing the spread of pandemic. The conceptual and technological platforms exploited are diverse, and it is likely that different vaccines will show to be better suited to distinct groups of the human population. Moreover, it remains to be elucidated whether and to what extent the capacity of vaccines under evaluation and of unrelated vaccines such as BCG can increase immunological fitness by training innate immunity to SARS-CoV-2 and pathogen-agnostic protection. Due to the short development time and the novelty of the technologies adopted, these vaccines will be deployed with several unresolved issues that only the passage of time will permit to clarify. Technical problems connected with the production of billions of doses and ethical ones connected with the availably of these vaccines also in the poorest countries, are imminent challenges facing us. It is our tenet that in the long run more than one vaccine will be needed to ensure equitable global access, protection of diverse subjects and immunity against viral variants.
Conflict of interest statement
GF declares no conflict of interest. AM has obtained lecture and consulting fees from Astra Zeneca, Pfizer and Janssen.
Figures


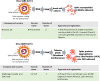


References
-
- Forni G, Mantovani A, Moretta L, Rezza G Vaccines. Accademia Nazionale dei Lincei. 2018. https://www.lincei.it/it/article/i-vaccini-vaccines-position-paper.
-
- WHO. Draft landscape of COVID-19 candidate vaccines. 2020. https://www.who.int/publications/m/item/draft-landscape-of-COVID-19-cand....
-
- Akst J. COVID-19 vaccine frontrunners. The Scientist. 2020. https://www.the-scientist.com/news-opinion/covid-19-vaccine-frontrunners....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

