Accelerated inflammatory aging in Alzheimer's disease and its relation to amyloid, tau, and cognition
- PMID: 33479445
- PMCID: PMC7820414
- DOI: 10.1038/s41598-021-81705-7
Accelerated inflammatory aging in Alzheimer's disease and its relation to amyloid, tau, and cognition
Abstract
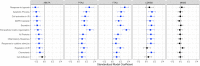
It is unclear how pathological aging of the inflammatory system relates to Alzheimer's disease (AD). We tested whether age-related inflammatory changes in cerebrospinal fluid (CSF) and plasma exist across different stages of AD, and whether such changes related to AD pathology. Linear regression was first used model chronological age in amyloid-β negative, cognitively unimpaired individuals (Aβ- CU; n = 312) based on a collection of 73 inflammatory proteins measured in both CSF and plasma. Fitted models were then applied on protein levels from Aβ+ individuals with mild cognitive impairment (Aβ+ MCI; n = 150) or Alzheimer's disease dementia (Aβ+ AD; n = 139) to test whether the age predicted from proteins alone ("inflammatory age") differed significantly from true chronological age. Aβ- individuals with subjective cognitive decline (Aβ- SCD; n = 125) or MCI (Aβ- MCI; n = 104) were used as an independent contrast group. The difference between inflammatory age and chronological age (InflammAGE score) was then assessed in relation to core AD biomarkers of amyloid, tau, and cognition. Both CSF and plasma inflammatory proteins were significantly associated with age in Aβ- CU individuals, with CSF-based proteins predicting chronological age better than plasma-based counterparts. Meanwhile, the Aβ- SCD and validation Aβ- CU groups were not characterized by significant inflammatory aging, while there was increased inflammatory aging in Aβ- MCI patients for CSF but not plasma inflammatory markers. Both CSF and plasma inflammatory changes were seen in the Aβ+ MCI and Aβ+ AD groups, with varying degrees of change compared to Aβ- CU and Aβ- SCD groups. Finally, CSF inflammatory changes were highly correlated with amyloid, tau, general neurodegeneration, and cognition, while plasma changes were mostly associated with amyloid and cognition. Inflammatory pathways change during aging and are specifically altered in AD, tracking closely with pathological hallmarks. These results have implications for tracking AD progression and for suggesting possible pathways for drug targeting.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Franke, K., Gaser, C. Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease 1data used in preparation of this article were obtained from the Alzheimer’s disease neuroimaging initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_.... Geropsych. 25, 235–245 (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

