Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA® trial
- PMID: 33479584
- PMCID: PMC7790983
- DOI: 10.1007/s13340-020-00447-5
Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA® trial
Abstract
Objective: Linagliptin, a dipeptidyl peptidase-4 inhibitor, recently demonstrated cardiovascular (CV) safety versus placebo in Asians with advanced type 2 diabetes mellitus (T2DM) in the CARMELINA® trial. We assessed its CV safety compared with the sulfonylurea glimepiride in Asians with relatively early T2DM in the CAROLINA® trial.
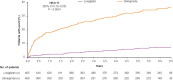
Methods: Based on prespecified and post hoc subgroup analyses of the multinational CAROLINA® trial in which adults with relatively early T2DM and elevated CV risk were randomized to linagliptin or glimepiride added to usual care, we analyzed data for participants from Asian countries. This included the primary outcome defined as time to first CV death, non-fatal myocardial infarction, or non-fatal stroke [three-point major adverse cardiovascular events (3P-MACE)].
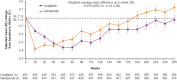
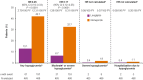
Results: Of the 6033 participants, 933 (15.5%) were from Asia. During a median follow-up of 6.2 years, 3P-MACE occurred in 9.5% and 11.1% of the linagliptin and glimepiride groups, respectively (hazard ratio [HR] 0.85; 95% confidence interval [CI] 0.57-1.26]), consistent with the overall population (HR 0.98; 95% CI 0.84-1.13; P = 0.17 for treatment by region interaction). Similarly, there were no significant differences between groups for other outcomes, including CV death (HR 0.73; 95% CI 0.38-1.38), non-CV mortality (HR 0.76; 95% CI 0.37-1.57) and hospitalization for heart failure (HR 0.89; 95% CI 0.36-2.19). Hypoglycemia adverse events occurred in 13.1% of linagliptin patients versus 42.1% of glimepiride patients (HR 0.25; 95% CI 0.19-0.33; P < 0.0001) despite similar glycemic control. Body weight was slightly lower with linagliptin relative to glimepiride: weighted average mean difference over 256 weeks of - 1.82 kg (95% CI - 2.28 to - 1.35).
Conclusions: In Asian patients, linagliptin demonstrated similar CV safety to glimepiride with a markedly lower rate of hypoglycemia and modestly lower weight.
Keywords: Cardiovascular; Diabetes mellitus, type 2.
© The Japan Diabetes Society 2020.
Conflict of interest statement
Conflict of interestTK reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca KK, Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kowa Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., SANWA KAGAKU KENKYUSHO CO., LTD., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited., and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Limited.; contracted research from AstraZeneca KK and Takeda Pharmaceutical Company Limited.; joint research from Daiichi Sankyo Company, Limited.; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited. JR has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Boehringer Ingelheim, and Intarcia; he has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon, Boehringer Ingelheim, and Intarcia. DY has received consulting or speaker fees from MSD KK, Novo Nordisk Pharma Ltd. and Taisho Toyama Pharmaceutical Co. Ltd., and clinically commissioned/joint research grants from Taisho Toyama Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Arklay Co. Ltd. and Terumo Co. Ltd. KK has received lecture fees from Boehringer Ingelheim, Eli Lilly and Sanofi. Boehringer Ingelheim, Mitsubishi Tanabe Pharma and Ono Pharmaceutical contributed to establishing the Division of Anticipatory Molecular Food Science and Technology, Medical Research Institute, Kanazawa Medical University, and is under contract for consultancy with Boehringer Ingelheim. GW, YP, YM have no conflicts to disclose. MM, AK, TO, and OEJ are employees of Boehringer Ingelheim. NM is funded by the German Research Foundation SFB TRR 219 (projects M-03 and M-05); reports giving lectures for and receiving honoraria from Amgen, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Lilly, Novo Nordisk; receiving unrestricted research grants from Boehringer Ingelheim; serving as an advisor for Amgen, Bayer, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Novo Nordisk; serving in trial leadership for Boehringer Ingelheim and Novo Nordisk; and declining all personal compensation from pharmaceutical and device companies.
Figures



References
-
- Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. - PubMed
-
- Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, Shaw JE, Zimmet PZ. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39(3):472–485. - PubMed
-
- Ramachandran A, Snehalatha C, Ma RC. Diabetes in South-East Asia: an update. Diabetes Res Clin Pract. 2014;103(2):231–237. - PubMed
-
- Lu J, Bi Y, Ning G. Curbing the obesity epidemic in China. Lancet Diabetes Endocrinol. 2016;4(6):470–471. - PubMed
LinkOut - more resources
Full Text Sources
