Small molecule inhibitors in pancreatic cancer
- PMID: 33479626
- PMCID: PMC7433757
- DOI: 10.1039/c9md00447e
Small molecule inhibitors in pancreatic cancer
Abstract
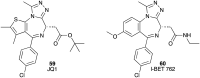
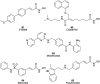
Pancreatic cancer (PC), with a 5 year survival of <7%, is one of the most fatal of all human cancers. The highly aggressive and metastatic character of this disease poses a challenge that current therapies are failing, despite significant efforts, to meet. This review examines the current status of the 35 small molecule inhibitors targeting pancreatic cancer in clinical trials and the >50 currently under investigation. These compounds inhibit biological targets spanning protein kinases, STAT3, BET, HDACs and Bcl-2 family proteins. Unsurprisingly, protein kinase inhibitors are overrepresented. Some trials show promise; a phase I combination trial of vorinostat 11 and capecitabine 17 gave a median overall survival (MoS) of 13 months and a phase II study of pazopanib 15 showed a MoS of 25 months. The current standard of care for metastatic pancreatic ductal adenocarcinoma, fluorouracil/folic acid (5-FU, Adrucil®), and gemcitabine (GEMZAR®) afforded a MoS of 23 and 23.6 months (EPAC-3 study), respectively. In patients who can tolerate the FOLFIRINOX regime, this is becoming the standard of treatment with a MoS of 11.1 months. Clinical study progress has been slow with limited improvement in patient survival relative to gemcitabine 1 monotherapy. A major cause of low PC survival is the late stage of diagnosis, occurring in patients who consider typical early stage warning signs of aches and pains normal. The selection of patients with specific disease phenotypes, the use of improved efficient drug combinations, the identification of biomarkers to specific cancer subtypes and more effective designs of investigation have improved outcomes. To move beyond the current dire condition and paucity of PC treatment options, determination of the best regimes and new treatment options is a challenge that must be met. The reasons for poor PC prognosis have remained largely unchanged for 20 years. This is arguably a consequence of significant changes in the drug discovery landscape, and the increasing pressure on academia to deliver short term 'media' friendly short-term news 'bites'. PC research sits at a pivotal point. Perhaps the greatest challenge is enacting a culture change that recognises that major breakthroughs are a result of blue sky, truly innovative and curiosity driven research.
This journal is © The Royal Society of Chemistry 2020.
Figures
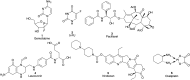



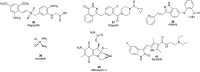
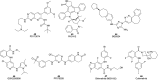
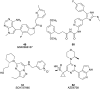






References
-
- Bailey P., Chang D. K., Nones K., Johns A. L., Patch A. M., Gingras M. C., Miller D. K., Christ A. N., Bruxner T. J., Quinn M. C., Nourse C., Murtaugh L. C., Harliwong I., Idrisoglu S., Manning S., Nourbakhsh E., Wani S., Fink L., Holmes O., Chin V., Anderson M. J., Kazakoff S., Leonard C., Newell F., Waddell N., Wood S., Xu Q., Wilson P. J., Cloonan N., Kassahn K. S., Taylor D., Quek K., Robertson A., Pantano L., Mincarelli L., Sanchez L. N., Evers L., Wu J., Pinese M., Cowley M. J., Jones M. D., Colvin E. K., Nagrial A. M., Humphrey E. S., Chantrill L. A., Mawson A., Humphris J., Chou A., Pajic M., Scarlett C. J., Pinho A. V., Giry-Laterriere M., Rooman I., Samra J. S., Kench J. G., Lovell J. A., Merrett N. D., Toon C. W., Epari K., Nguyen N. Q., Barbour A., Zeps N., Moran-Jones K., Jamieson N. B., Graham J. S., Duthie F., Oien K., Hair J., Grützmann R., Maitra A., Iacobuzio-Donahue C. A., Wolfgang C. L., Morgan R. A., Lawlor R. T., Corbo V., Bassi C., Rusev B., Capelli P., Salvia R., Tortora G., Mukhopadhyay D., Petersen G. M., Initiative Australian Pancreatic Cancer Genome, Munzy D. M., Fisher W. F., Karim S. A., Eshleman J. R., Hruban R. H., Pilarsky C., Morton J. P., Sansom O. J., Scarpa A., Musgrove E. A., Bailey U. M., Hofmann O., Sutherland R. L., Wheeler D. A., Gill A. J., Gibbs R. A., Pearson J. V., Waddell N., Biankin A. V., Grimmond S. M. Nature. 2016;531:47–52. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

