Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer
- PMID: 33479648
- PMCID: PMC7580774
- DOI: 10.1039/c9md00570f
Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer
Abstract
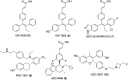
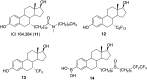
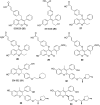
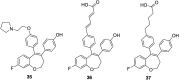
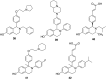
Selective estrogen receptor downregulators (SERDs) are a novel class of compounds capable of reducing the ERα protein level and blocking ER activity. Therefore, SERDs are considered as a significant therapeutic approach to treat ER+ breast cancer in both early stage and more advanced drug-resistant cases. After the FDA approval of a steroidal drug, fulvestrant, as a SERD for the treatment of breast cancer in patients who have progressed on antihormonal agents, several molecules with diverse chemical structures have been rapidly developed, studied and evaluated for selective estrogen receptor downregulation activity. Here we compile the promising SERDs reported in recent years and discuss the chemical structure and pharmacological profile of the most potent compound of the considered series. Because of the availability of only a limited number of effective drugs for the treatment of breast cancer, the quest for a potent SERD with respectable activity and bioavailability is still ongoing. The goal of this article is to make available to the reader an overview of the current progress in SERDs and provide clues for the future discovery and development of novel pharmacological potent SERDs for the treatment of breast cancer.
This journal is © The Royal Society of Chemistry 2020.
Figures
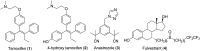






References
-
- World Health Organisation, Cancer: Diagnosis and Treatment. https://www.who.int/cancer/treatment/en/, 2017.
-
- Tryfonidis K., Zardavas D., Katzenellenbogen B. S., Piccart M. Cancer Treat. Rev. 2016;50:68e81. - PubMed
-
- Shagufta, Ahmad I. Eur. J. Med. Chem. 2018;143:515–531. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

