An investigation of the antileishmanial properties of semi-synthetic saponins
- PMID: 33479679
- PMCID: PMC7651632
- DOI: 10.1039/d0md00123f
An investigation of the antileishmanial properties of semi-synthetic saponins
Abstract
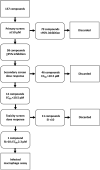
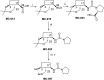
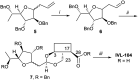
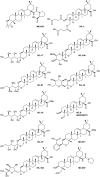
Leishmaniasis is a neglected tropical disease caused by insect-vector borne protozoan parasites of the, Leishmania species. Whilst infection threatens and affects millions of the global poor, vaccines are absent and drug therapy limited. Extensive efforts have recently been made to discover new leads from small molecule synthetic compound libraries held by industry; however, the number of new chemical entities identified and entering development as anti-leishmanials has been very low. This has led to increased interest in the possibility of discovering naturally derived compounds with potent antileishmanial activity which may be developed towards clinical applications. Plant-derived triterpenoid and steroidal saponins have long been considered as anti-microbials and here we describe an investigation of a library of 137 natural (9) and semi-synthetic saponins (128) for activity against Leishmania mexicana, a causative agent of cutaneous leishmaniasis. The triterpenoid sapogenin, hederagenin, readily obtained in large quantities from Hedera helix (common ivy), was converted into a range of 128 derivatives. These semi-synthetic compounds, as well as saponins isolated from ivy, were examined with a phenotypic screening approach to identify potent and selective anti-leishmanial hits. This led to the identification of 12 compounds, including the natural saponin gypsogenin, demonstrating high potency (ED50 < 10.5 μM) against axenic L. mexicana amastigotes, the mammalian pathogenic form. One of these, hederagenin disuccinate, was sufficiently non-toxic to the macrophage host cell to facilitate further analyses, selectivity index (SI) > 10. Whilst this was not active in an infected cell model, the anti-leishmanial properties of hederagenin-derivatives have been demonstrated, and the possibility of improving the selectivity of natural hederagenin through chemical modification has been established.
This journal is © The Royal Society of Chemistry 2020.
Figures
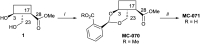



References
-
- WHO, https://www.who.int/leishmaniasis/en/, 2019.
-
- Reithinger R., Dujardin J. C., Louzir H., Pirmez C., Alexander B., Brooker S. Lancet Infect. Dis. 2007;7:581–596. - PubMed
-
- Burza S., Croft S. L., Boelaert M. Lancet. 2018;392:951–970. - PubMed
-
- Khare S., Nagle A. S., Biggart A., Lai Y. H., Liang F., Davis L. C., Barnes S. W., Mathison C. J., Myburgh E., Gao M. Y., Gillespie J. R., Liu X., Tan J. L., Stinson M., Rivera I. C., Ballard J., Yeh V., Groessl T., Federe G., Koh H. X., Venable J. D., Bursulaya B., Shapiro M., Mishra P. K., Spraggon G., Brock A., Mottram J. C., Buckner F. S., Rao S. P., Wen B. G., Walker J. R., Tuntland T., Molteni V., Glynne R. J., Supek F. Nature. 2016;537:229–233. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

