4-Nitrophenyl activated esters are superior synthons for indirect radiofluorination of biomolecules
- PMID: 33479687
- PMCID: PMC7517343
- DOI: 10.1039/d0md00140f
4-Nitrophenyl activated esters are superior synthons for indirect radiofluorination of biomolecules
Abstract
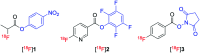
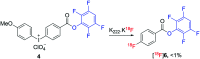
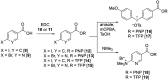
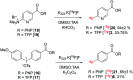
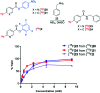
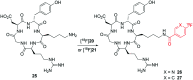
Indirect radiolabelling has for a long time been the mainstay strategy for radiofluorination of biomolecules. Acylation of biomolecules through the use of an 18F-labelled activated ester is a standard method for indirect radiolabelling. However, the preparation of 18F-labelled activated esters is typically a complex and multistep procedure. Herein, we describe the use of 4-nitrophenyl (PNP) activated esters to rapidly prepare 18F-labelled acylation synthons in one step. Furthermore, we present a comparative study of PNP activated esters and the commonly utilised 2,3,5,6-tetrafluorphenyl (TFP) activated esters under direct radiofluorination conditions and demonstrate their relative acylation behaviour. We demonstrate the superiority of PNP esters under direct radiofluorination conditions with favourable acylation kinetics.
This journal is © The Royal Society of Chemistry 2020.
Figures
References
-
- Massoud T. F., Gambhir S. S. Genes Dev. 2003;17:545–580. - PubMed
-
- Pither R. Expert Rev. Mol. Diagn. 2003;3:703–713. - PubMed
-
- Gulyás B., Halldin C. Q. J. Nucl. Med. Mol. Imaging. 2012;56:173–190. - PubMed
-
- Fani M., André J. P., Maecke H. R. Contrast Media Mol. Imaging. 2008;3:53–63. - PubMed
-
- Okarvi S. Eur. J. Nucl. Med. 2001;28:929–938. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

