A Phase 3, Randomized, Investigator-blinded Trial Comparing Ceftobiprole With a Standard-of-care Cephalosporin, With or Without Vancomycin, for the Treatment of Pneumonia in Pediatric Patients
- PMID: 33480665
- PMCID: PMC8104010
- DOI: 10.1097/INF.0000000000003077
A Phase 3, Randomized, Investigator-blinded Trial Comparing Ceftobiprole With a Standard-of-care Cephalosporin, With or Without Vancomycin, for the Treatment of Pneumonia in Pediatric Patients
Abstract
Background: The advanced-generation, broad-spectrum, intravenous (IV) cephalosporin, ceftobiprole, is an effective and well-tolerated treatment for adults with hospital-acquired pneumonia (HAP) or community-acquired pneumonia (CAP), but its effects in pediatric patients have not been established.
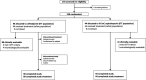
Methods: In this multicenter, investigator-blinded, active-controlled, phase 3 study, patients 3 months to <18 years old with HAP or CAP requiring hospitalization were randomized (2:1) to ceftobiprole versus standard-of-care (SoC) IV cephalosporin treatments (ceftazidime or ceftriaxone), with or without vancomycin. After at least 3 days' IV treatment, patients demonstrating clinical improvement could be switched to an oral antibiotic, to complete a minimum of 7 days' treatment.
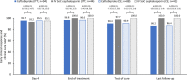
Results: Overall, 138 patients were randomized to ceftobiprole (n = 94) or a SoC cephalosporin (n = 44). Median time to oral switch was 6.0 days in the ceftobiprole group and 8.0 days in the SoC cephalosporin group. While on IV therapy, adverse events and treatment-related adverse events were reported by 20.2% and 8.5% of ceftobiprole-treated patients and 18.2% and 0% of SoC cephalosporin-treated patients. Early clinical response rates at day 4 in the intention-to-treat population were 95.7% and 93.2% (between-group difference, 2.6%; 95% confidence interval, -5.5% to 14.7%) in the ceftobiprole and comparator groups, and clinical cure rates at the test-of-cure visit were 90.4% and 97.7% (between-group difference, -7.3%; 95% confidence interval, -15.7% to 3.6%), respectively.
Conclusions: Ceftobiprole was well tolerated and, in this small phase 3 study, demonstrated similar efficacy to SoC cephalosporins in pediatric patients with HAP or CAP requiring hospitalization.
Trial registration: ClinicalTrials.gov NCT03439124.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.S., J.I.S. and K.A.H. are employees of Basilea Pharmaceutica International Ltd. The other authors have no conflicts of interest to disclose.
Figures

References
-
- Liu L, Oza S, Hogan D, et al. . Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. - PubMed
-
- Madhi SA, De Wals P, Grijalva CG, et al. . The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J. 2013;32:e119–e127. - PubMed
-
- Harris M, Clark J, Coote N, et al. . British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1. - PubMed
-
- Rudan I, O’Brien KL, Nair H, et al. ; Child Health Epidemiology Reference Group (CHERG). Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

