Hormonal Therapy for Prostate Cancer
- PMID: 33480983
- PMCID: PMC8152444
- DOI: 10.1210/endrev/bnab002
Hormonal Therapy for Prostate Cancer
Abstract
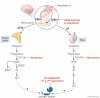
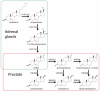
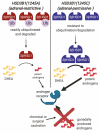
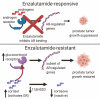
Huggins and Hodges demonstrated the therapeutic effect of gonadal testosterone deprivation in the 1940s and therefore firmly established the concept that prostate cancer is a highly androgen-dependent disease. Since that time, hormonal therapy has undergone iterative advancement, from the types of gonadal testosterone deprivation to modalities that block the generation of adrenal and other extragonadal androgens, to those that directly bind and inhibit the androgen receptor (AR). The clinical states of prostate cancer are the product of a superimposition of these therapies with nonmetastatic advanced prostate cancer, as well as frankly metastatic disease. Today's standard of care for advanced prostate cancer includes gonadotropin-releasing hormone agonists (e.g., leuprolide), second-generation nonsteroidal AR antagonists (enzalutamide, apalutamide, and darolutamide) and the androgen biosynthesis inhibitor abiraterone. The purpose of this review is to provide an assessment of hormonal therapies for the various clinical states of prostate cancer. The advancement of today's standard of care will require an accounting of an individual's androgen physiology that also has recently recognized germline determinants of peripheral androgen metabolism, which include HSD3B1 inheritance.
Keywords: abiraterone; androgen deprivation therapy; androgens; enzalutamide; glucocorticoids; prostate cancer; steroids.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. - PubMed
-
- SEER*Explorer: an interactive website for SEER cancer statistics. Surveillance Research Program. https://seer.cancer.gov. Accessed February 14, 2021.
-
- Resnick MJ, Penson DF. Quality of life with advanced metastatic prostate cancer. Urol Clin North Am. 2012;39(4):505-515. - PubMed
-
- Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

