Exploration for the real causative agents of licorice-induced pseudoaldosteronism
- PMID: 33481180
- PMCID: PMC7902566
- DOI: 10.1007/s11418-021-01484-3
Exploration for the real causative agents of licorice-induced pseudoaldosteronism
Abstract
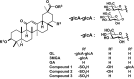
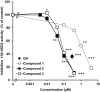
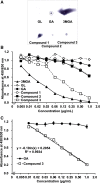
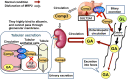
I investigated the causative agents of licorice-induced pseudoaldosteronism, which is a frequent side effect of Japanese traditional Kampo medicines. Glycyrrhizin (GL), the main ingredient of licorice, is absorbed after being metabolized to glycyrrhetinic acid (GA) by intestinal bacteria, and then metabolized in liver to 3-monoglucuronyl-glycyrrhetinic acid (3MGA). In normal condition, 3MGA is excreted into bile via a multidrug resistance-related protein (Mrp) 2, therefore, 3MGA does not appear in blood circulation. However, under the dysfunction of Mrp2, 3MGA appears in the blood circulation and is excreted into the urine by not glomerular filtration but tubular secretion via organic anion transporter (OAT) 1 and 3. At this time, 3MGA inhibits type 2 11β-hydroxysteroid dehydrogenase (11βHSD2) in tubular cells to cause pseudoaldosteronism. Since GA is not the substrates of these transporters, GA cannot inhibit 11βHSD2 in tubular cells. Therefore, it was considered that 3MGA was the causative agents of licorice-induced pseudoaldosteronism. After that, I isolated and identified three other GL metabolites, 22α-hydroxy-18β-glycyrrhetyl-3-O-sulfate-30-glucuronide (1), 22α-hydroxy-18β-glycyrrhetyl-3-O-sulfate (2), and 18β-glycyrrhetyl-3-O-sulfate (3) from the urine of Mrp2-deficient rats orally treated with GA, and found that their blood and urinary concentrations were much higher than 3MGA and that their pharmacokinetic behaviors were similar to 3MGA. 3MGA was not detected in the blood of patients with pseudoaldosteronism who developed rhabdomyolysis due to licorice, and compound 3 was detected at a high concentration. In addition, a multicenter retrospective study was conducted using the serum and urine of 97 patients who took Kampo medicines containing licorice. Of a total of 97 patients, 67 detected GA in the serum (median 122 nM, 5 nM-1.8 µM) and 68 detected compound 3 (median 239 nM, 2 nM-4.2 µM), and there were no cases of detection of GL, 3MGA, compounds 1, and 2. High blood concentrations of compound 3 were associated with low plasma renin activity, plasma aldosterone levels, and serum potassium levels. It is highly probable that compound 3 is the true causative agent of pseudoaldosteronism.
Keywords: 18β-glycyrrhetyl-3-O-sulfate; Adverse effects; Glycyrrhiza; Glycyrrhizin; Licorice; Pseudoaldosteronism.
Figures


Similar articles
-
18β-glycyrrhetyl-3-O-sulfate would be a causative agent of licorice-induced pseudoaldosteronism.Sci Rep. 2019 Feb 7;9(1):1587. doi: 10.1038/s41598-018-38182-2. Sci Rep. 2019. PMID: 30733510 Free PMC article.
-
3-Monoglucuronyl glycyrrhretinic acid is a possible marker compound related to licorice-induced pseudoaldosteronism.Biol Pharm Bull. 2014;37(6):898-902. doi: 10.1248/bpb.b13-00997. Biol Pharm Bull. 2014. PMID: 24882402 Review.
-
Identification of glycyrrhizin metabolites in humans and of a potential biomarker of liquorice-induced pseudoaldosteronism: a multi-centre cross-sectional study.Arch Toxicol. 2019 Nov;93(11):3111-3119. doi: 10.1007/s00204-019-02588-2. Epub 2019 Oct 11. Arch Toxicol. 2019. PMID: 31605160
-
3-Monoglucuronyl-glycyrrhretinic acid is a substrate of organic anion transporters expressed in tubular epithelial cells and plays important roles in licorice-induced pseudoaldosteronism by inhibiting 11β-hydroxysteroid dehydrogenase 2.J Pharmacol Exp Ther. 2012 Aug;342(2):297-304. doi: 10.1124/jpet.111.190009. Epub 2012 Apr 27. J Pharmacol Exp Ther. 2012. PMID: 22543032
-
Clinical Risk Factors of Licorice-Induced Pseudoaldosteronism Based on Glycyrrhizin-Metabolite Concentrations: A Narrative Review.Front Nutr. 2021 Sep 17;8:719197. doi: 10.3389/fnut.2021.719197. eCollection 2021. Front Nutr. 2021. PMID: 34604277 Free PMC article. Review.
Cited by
-
Application of Monoclonal Antibodies against Naturally Occurring Bioactive Ingredients.Antibodies (Basel). 2024 Jul 24;13(3):60. doi: 10.3390/antib13030060. Antibodies (Basel). 2024. PMID: 39189231 Free PMC article. Review.
-
Targeting microglia polarization with Chinese herb-derived natural compounds for neuroprotection in ischemic stroke.Front Cell Dev Biol. 2025 Jun 10;13:1580479. doi: 10.3389/fcell.2025.1580479. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40556734 Free PMC article. Review.
-
Treatment with Glycyrrhiza glabra Extract Induces Anxiolytic Effects Associated with Reduced Salt Preference and Changes in Barrier Protein Gene Expression.Nutrients. 2024 Feb 13;16(4):515. doi: 10.3390/nu16040515. Nutrients. 2024. PMID: 38398838 Free PMC article.
-
Analysis of clinical factors associated with Kampo formula-induced pseudoaldosteronism based on self-reported information from the Japanese Adverse Drug Event Report database.PLoS One. 2024 Jan 2;19(1):e0296450. doi: 10.1371/journal.pone.0296450. eCollection 2024. PLoS One. 2024. PMID: 38165850 Free PMC article.
-
Kampo medicines for supportive care of patients with cancer: A brief review.Integr Med Res. 2022 Jun;11(2):100839. doi: 10.1016/j.imr.2022.100839. Epub 2022 Feb 23. Integr Med Res. 2022. PMID: 35242536 Free PMC article. Review.
References
-
- United States Pharmacopeial Convention . United Sates Pharmacopeia 43–National Formulary 38. North Bethesda, Maryland, United States: United States Pharmacopeial Convention; 2020.
-
- Pharmaceutical and Medical Device Regulatory Science Society of Japan . Japanese pharmacopoeia seventeenth edition (JP XVII) Tokyo: Jiho; 2016.
-
- Japan Kampo Medicines Manufacturers’ Association under the super vision of National Institute of Health Sciences, the affiliated institutions of the Ministry of Health, Labour and Welfare of Japan . Handbook on OTC medicinal products in Kampo. Tokyo: Jiho; 2013.
-
- Morimoto Y, Nakajima C. Pseudoaldosteronism induced by licorice derivatives in Japan. J Trad Med. 1991;8:1–22.
Publication types
MeSH terms
Substances
Grants and funding
- JP20790475/Japan Society for the Promotion of Science
- JP23790748,/Japan Society for the Promotion of Science
- JP25460907/Japan Society for the Promotion of Science
- JP17lk0310036h0001/Japan Agency for Medical Research and Development
- JP18lk0310049h0001/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Other Literature Sources

