An Alternative to Carbon Additives: The Fabrication of Conductive Layers Enabled by Soluble Conducting Polymer Precursors - A Case Study for Organic Batteries
- PMID: 33481558
- PMCID: PMC7877702
- DOI: 10.1021/acsami.0c22578
An Alternative to Carbon Additives: The Fabrication of Conductive Layers Enabled by Soluble Conducting Polymer Precursors - A Case Study for Organic Batteries
Abstract
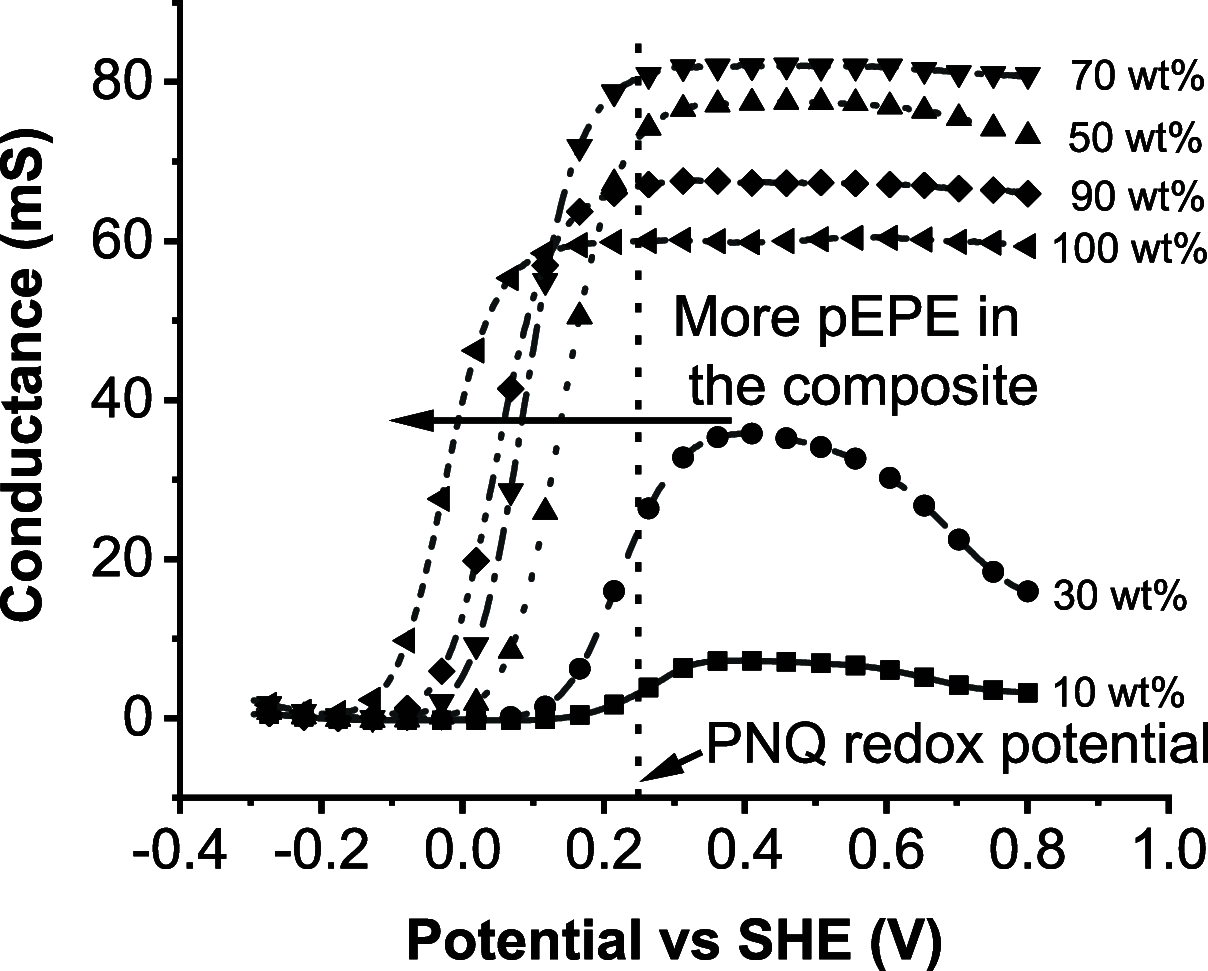
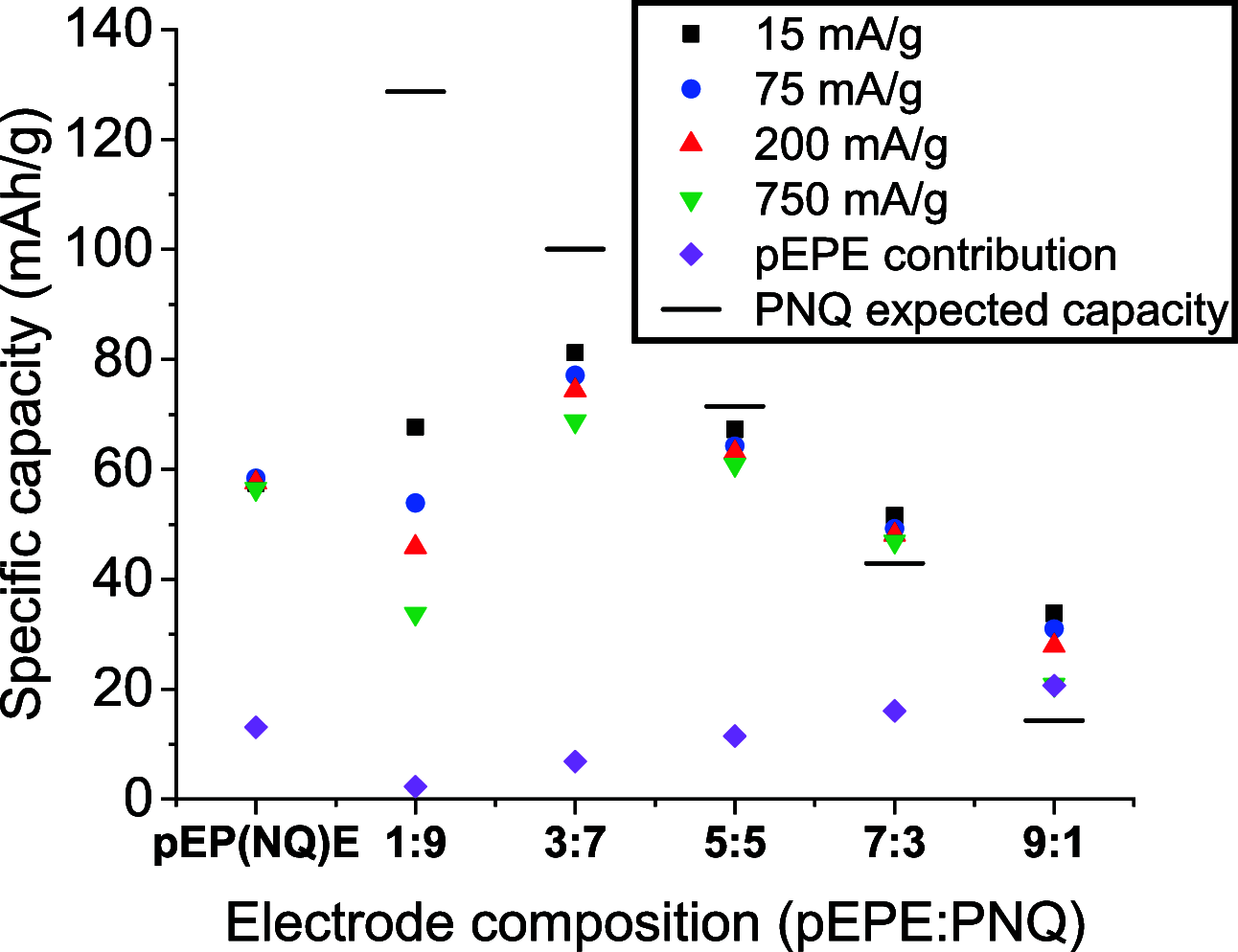
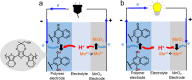
Utilizing organic redox-active materials as electrodes is a promising strategy to enable innovative battery designs with low environmental footprint during production, which can be hard to achieve with traditional inorganic materials. Most electrode compositions, organic or inorganic, require binders for adhesion and conducting additives to enable charge transfer through the electrode, in addition to the redox-active material. Depending on the redox-active material, many types and combinations of binders and conducting additives have been considered. We designed a conducting polymer (CP), with a soluble, trimeric unit based on 3,4-ethylenedioxythiophene (E) and 3,4-propylenedioxythiophene (P) as the repeat unit, acting as a combined binder and conducting additive. While CPs as additives have been explored earlier, in the current work, the use of a trimeric precursor enables solution processing together with the organic redox-active material. To evaluate this concept, the CP was blended with a redox polymer (RP), which contained a naphthoquinone (NQ) redox group at different ratios. The highest capacity for the total weight of the CP/RP electrode was 77 mAh/g at 1 C in the case of 30% EPE and 70% naphthoquinone-substituted poly(allylamine) (PNQ), which is 70% of the theoretical capacity given by the RP in the electrode. We further used this electrode in an aqueous battery, with a MnSO4 cathode. The battery displayed a voltage of 0.95 V, retaining 93% of the initial capacity even after 500 cycles at 1 C. The strategy of using a solution-processable CP precursor opens up for new organic battery designs and facile evaluation of RPs in such.
Keywords: conducting polymers; conductivity additives; organic battery; organic electrode; quinones; redox-active polymer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Poizot P.; Dolhem F.; Gaubicher J. Progress in All-Organic Rechargeable Batteries Using Cationic and Anionic Configurations: Toward Low-Cost and Greener Storage Solutions?. Curr. Opin. Electrochem. 2018, 9, 70–80. 10.1016/j.coelec.2018.04.003. - DOI
-
- Poizot P.; Dolhem F. Clean Energy New Deal for a Sustainable World: From Non-Co2 Generating Energy Sources to Greener Electrochemical Storage Devices. Energy Environ. Sci. 2011, 4, 2003–2019. 10.1039/C0EE00731E. - DOI
-
- Casado N.; Hernández G.; Sardon H.; Mecerreyes D. Current Trends in Redox Polymers for Energy and Medicine. Prog. Polym. Sci. 2016, 52, 107–135. 10.1016/j.progpolymsci.2015.08.003. - DOI
-
- Oka K.; Kato R.; Oyaizu K.; Nishide H. Poly(Vinyldibenzothiophenesulfone): Its Redox Capability at Very Negative Potential toward an All-Organic Rechargeable Device with High-Energy Density. Adv. Funct. Mater. 2018, 28, 1805858.10.1002/adfm.201805858. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

