First Characterization of the Formation of Anthocyanin-Ge and Anthocyanin-B Complexes through UV-Vis Spectroscopy and Density Functional Theory Quantum Chemical Calculations
- PMID: 33481589
- PMCID: PMC7875511
- DOI: 10.1021/acs.jafc.0c06827
First Characterization of the Formation of Anthocyanin-Ge and Anthocyanin-B Complexes through UV-Vis Spectroscopy and Density Functional Theory Quantum Chemical Calculations
Abstract
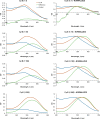
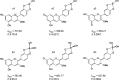
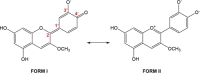
The occurrence of anthocyanin (ACN) and metal (Me) complexes has been widely supported by many research works while the possibility that ACNs bind to metalloids (Mds) is yet to be proven. Here, metalloids (H3BO3 for B; GeO2 for Ge) were added to cyanidin-based solutions at pH 5, 6, and 7 and ACN-Md stoichiometric ratios of 1:1, 1:10, 1:100, and 1:500, and UV-vis transmittance spectroscopy as well as density functional theory (DFT) calculations were performed to test this hypothesis. Ge and B addition caused bathochromic and hyperchromic shifts on ACN UV-vis spectra, particularly pronounced at pH 5 and a 1:500 (ACN:Md) ratio. ACN-Me complexation reactions have been evaluated where Ge showed a higher capability to bind to ACNs than B. Among the complexes envisioned, those labeled as b1, b2, and b3 feature UV-vis spectra compatible with experiments. The combination of experimental and computational data offers for the first time evidence of the formation of ACN-Md complexes.
Keywords: anthocyanin−metalloid complex; bathochromic shift; hyperchromic effect; molecular absorption simulations; molecular modeling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Landi M.; Tattini M.; Gould K. S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. 10.1016/j.envexpbot.2015.05.012. - DOI
-
- Hatier J. H. B.; Gould K. S.. Anthocyanin function in vegetative organs. In Anthocyanins: Biosynthesis, Functions, and Applications; Gould K. S., Davies K. M., Winefield C., Eds.; Springer: New York, 2009; pp 1–20.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

