Contrasting mechanisms for hidden hearing loss: Synaptopathy vs myelin defects
- PMID: 33481777
- PMCID: PMC7857583
- DOI: 10.1371/journal.pcbi.1008499
Contrasting mechanisms for hidden hearing loss: Synaptopathy vs myelin defects
Erratum in
-
Correction: Contrasting mechanisms for hidden hearing loss: Synaptopathy vs myelin defects.PLoS Comput Biol. 2021 Apr 7;17(4):e1008910. doi: 10.1371/journal.pcbi.1008910. eCollection 2021 Apr. PLoS Comput Biol. 2021. PMID: 33826606 Free PMC article.
Abstract
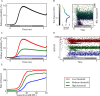
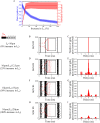
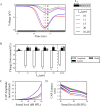
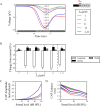
Hidden hearing loss (HHL) is an auditory neuropathy characterized by normal hearing thresholds but reduced amplitudes of the sound-evoked auditory nerve compound action potential (CAP). In animal models, HHL can be caused by moderate noise exposure or aging, which induces loss of inner hair cell (IHC) synapses. In contrast, recent evidence has shown that transient loss of cochlear Schwann cells also causes permanent auditory deficits in mice with similarities to HHL. Histological analysis of the cochlea after auditory nerve remyelination showed a permanent disruption of the myelination patterns at the heminode of type I spiral ganglion neuron (SGN) peripheral terminals, suggesting that this defect could be contributing to HHL. To shed light on the mechanisms of different HHL scenarios observed in animals and to test their impact on type I SGN activity, we constructed a reduced biophysical model for a population of SGN peripheral axons whose activity is driven by a well-accepted model of cochlear sound processing. We found that the amplitudes of simulated sound-evoked SGN CAPs are lower and have greater latencies when heminodes are disorganized, i.e. they occur at different distances from the hair cell rather than at the same distance as in the normal cochlea. These results confirm that disruption of heminode positions causes desynchronization of SGN spikes leading to a loss of temporal resolution and reduction of the sound-evoked SGN CAP. Another mechanism resulting in HHL is loss of IHC synapses, i.e., synaptopathy. For comparison, we simulated synaptopathy by removing high threshold IHC-SGN synapses and found that the amplitude of simulated sound-evoked SGN CAPs decreases while latencies remain unchanged, as has been observed in noise exposed animals. Thus, model results illuminate diverse disruptions caused by synaptopathy and demyelination on neural activity in auditory processing that contribute to HHL as observed in animal models and that can contribute to perceptual deficits induced by nerve damage in humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Binaural Processing Deficits Due to Synaptopathy and Myelin Defects.Front Neural Circuits. 2022 Apr 14;16:856926. doi: 10.3389/fncir.2022.856926. eCollection 2022. Front Neural Circuits. 2022. PMID: 35498371 Free PMC article.
-
Transient auditory nerve demyelination as a new mechanism for hidden hearing loss.Nat Commun. 2017 Feb 17;8:14487. doi: 10.1038/ncomms14487. Nat Commun. 2017. PMID: 28211470 Free PMC article.
-
Biased auditory nerve central synaptopathy is associated with age-related hearing loss.J Physiol. 2021 Mar;599(6):1833-1854. doi: 10.1113/JP281014. Epub 2021 Jan 31. J Physiol. 2021. PMID: 33450070 Free PMC article.
-
Hidden Hearing Loss: A Disorder with Multiple Etiologies and Mechanisms.Cold Spring Harb Perspect Med. 2020 Jan 2;10(1):a035493. doi: 10.1101/cshperspect.a035493. Cold Spring Harb Perspect Med. 2020. PMID: 30617057 Free PMC article. Review.
-
Noise-induced cochlear synaptopathy: Past findings and future studies.Hear Res. 2017 Jun;349:148-154. doi: 10.1016/j.heares.2016.12.008. Epub 2016 Dec 19. Hear Res. 2017. PMID: 28007526 Review.
Cited by
-
Conditional Ablation of Glucocorticoid and Mineralocorticoid Receptors from Cochlear Supporting Cells Reveals Their Differential Roles for Hearing Sensitivity and Dynamics of Recovery from Noise-Induced Hearing Loss.Int J Mol Sci. 2023 Feb 7;24(4):3320. doi: 10.3390/ijms24043320. Int J Mol Sci. 2023. PMID: 36834731 Free PMC article.
-
N-acetylcysteine microparticles reduce cisplatin-induced RSC96 Schwann cell toxicity.Laryngoscope Investig Otolaryngol. 2024 May 17;9(3):e1256. doi: 10.1002/lio2.1256. eCollection 2024 Jun. Laryngoscope Investig Otolaryngol. 2024. PMID: 38765675 Free PMC article.
-
The role of GDF15 in attenuating noise-induced hidden hearing loss by alleviating oxidative stress.Cell Biol Toxicol. 2024 Sep 18;40(1):79. doi: 10.1007/s10565-024-09912-2. Cell Biol Toxicol. 2024. PMID: 39289208 Free PMC article.
-
Hidden hearing loss in a Charcot-Marie-Tooth type 1A mouse model.JCI Insight. 2024 Oct 8;9(19):e180315. doi: 10.1172/jci.insight.180315. JCI Insight. 2024. PMID: 39178128 Free PMC article.
-
The human OPA1delTTAG mutation induces adult onset and progressive auditory neuropathy in mice.Cell Mol Life Sci. 2024 Feb 9;81(1):80. doi: 10.1007/s00018-024-05115-4. Cell Mol Life Sci. 2024. PMID: 38334784 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

