Clinical and pharmacokinetic/dynamic outcomes of prolonged infusions of beta-lactam antimicrobials: An overview of systematic reviews
- PMID: 33481817
- PMCID: PMC7822342
- DOI: 10.1371/journal.pone.0244966
Clinical and pharmacokinetic/dynamic outcomes of prolonged infusions of beta-lactam antimicrobials: An overview of systematic reviews
Abstract
Objective: This overview of reviews aims to map and compare of objectives, methods, and findings of existing systematic reviews to develop a greater understanding of the information available about prolonged beta-lactam infusions in hospitalized patients with infection.
Design: Overview of systematic reviews.
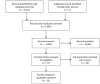
Data sources: Medline, Embase, PROSPERO and the Cochrane Library were systematically searched from January, 1990 to June, 2019 using a peer reviewed search strategy. Grey literature was also searched for relevant reviews.
Eligibility criteria for selecting reviews: Systematic reviews were sought that compared two or more infusion strategies for intravenous beta-lactam antimicrobials and report clinical cure or mortality. Populations of included reviews were restricted to hospitalized patients with infection, without restrictions on age, infection type, or disease.
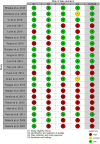
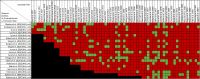
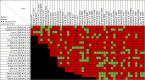
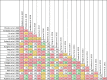
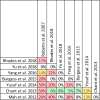
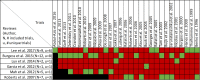
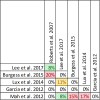
Data extraction and analysis: Abstract screening, data extraction, quality and risk of bias assessment were conducted by two independent reviewers. Overlap between reviews was assessed using a modified corrected covered area. Overview findings are reported in accordance with Cochrane's recommendation for overview conduct. Clinical outcomes extracted included survival, clinical cure, treatment failure, microbiological cure, length of stay, adverse events, cost, and emergence of resistance.
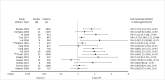
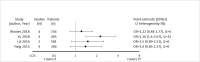
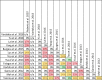
Results: The search strategy identified 3327 unique citations from which 21 eligible reviews were included. Reviews varied by population, intervention and outcomes studied. Between reviews, overlap of primary studies was generally high, methodologic quality generally low and risk of bias variable. Nine of 14 reviews that quantitatively evaluated mortality and clinical cure identified a benefit with prolonged infusions of beta lactams when compared with intermittent infusions. Evidence of mortality and clinical cure benefit was greater among critically ill patients when compared to less sick patients and lower in randomized controlled trials when compared with observational studies.
Conclusions: Findings from our review demonstrate a consistent and reproducible lack of harm with prolonged infusions of beta-lactam antibiotics with variability in effect size and significance of benefits. Despite 21 systematic reviews addressing prolonged infusions of beta-lactams, this overview supports the continued need for a definitive systematic review given variability in populations, interventions and outcomes in the current systematic reviews. Subsequent systematic reviews should have more rigorous and transparent methods, only include RCTs and evaluate the proposed benefits found in various subgroup-analyses-i.e. high risk of mortality.
Trial registration: Prospero registry, CRD42019117118.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

